Portal:Chemistry/Featured article
Usage
[edit]The layout design for these subpages is at Portal:Chemistry/Featured article/Layout.
- Add a new Featured article to the next available subpage.
- Update "max=" to new total for its {{Random portal component}} on the main page.
Featured articles list
[edit]Portal:Chemistry/Featured article/1
The chemical substance hydrochloric acid is the aqueous (water-based) solution of hydrogen chloride (HCl) gas. It is a strong acid, the major component of gastric acid and of wide industrial use. As a highly corrosive liquid, hydrochloric acid should be handled only with appropriate safety precautions.
Hydrochloric acid, or muriatic acid by its historical but still occasionally used name, has been an important and frequently used chemical from early history, and was discovered by the alchemist Jābir ibn Hayyān around 800. It was used throughout the Middle Ages by alchemists in the quest for the philosopher's stone, and later by several European scientists including Glauber, Priestley, and Davy, to help establish modern chemical knowledge.
During the Industrial Revolution, it became an important industrial chemical for many applications, including the large scale production of organic compounds such as vinyl chloride for PVC plastic and MDI/TDI for polyurethane and smaller scale applications, such as production of gelatin and other ingredients in food, and leather processing. Currently, production is approximately 20 million metric tonnes annually (20 Mt/year) of HCl gas.
Portal:Chemistry/Featured article/2
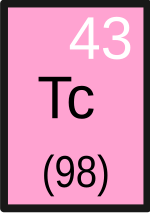
Technetium is a chemical element that has the symbol Tc and the atomic number 43. Pronounced tek-nee-s(h)ee-um, the chemical properties of this silvery grey, radioactive, crystalline transition metal are intermediate between rhenium and manganese. Its short-lived isotope Tc-99m is used in nuclear medicine for a wide variety of diagnostic tests. Tc-99 is used as a gamma ray-free source of beta particles, and its pertechnetate ion (TcO−
4) could find use as an anodic corrosion preventer for steel (this possible use is hindered by technetium's radioactivity). The pertechnetate forms a technetium dioxide layer on the steel surface, this TcO2 formation explains how iron powder can be used to remove technetium from water. It is also possible to absorb technetium on activated carbon.
Dmitri Mendeleev predicted many of the properties of element 43, which he called ekamanganese, well before its actual discovery (see Mendeleev's predicted elements). In 1937 its isotope Tc-97 became the first element to be artificially produced, hence its name (from the Greek τεχνητος, meaning "artificial"). Most technetium produced on Earth is a by-product of fission of uranium-235 in nuclear reactors and is extracted from nuclear fuel rods. No isotope of technetium has a half-life longer than 4.2 million years (Tc-98), so its detection in red giants in 1952 helped bolster the theory that stars can produce heavier elements. On earth, technetium occurs naturally only in uranium ores as a product of spontaneous fission; the quantities are minute but have been measured.
Portal:Chemistry/Featured article/3
Titanium is a chemical element in the periodic table that has the symbol Ti and atomic number 22. It is a light, strong, lustrous, corrosion-resistant (including resistance to sea water and chlorine) transition metal with a white-silvery-metallic colour. Titanium is used in strong light-weight alloys (most notably with iron and aluminium) and its most common compound, titanium dioxide, is used in white pigments. Substances containing titanium are called titaniferous.
This element occurs in numerous minerals with the main sources being rutile and ilmenite, which are widely distributed over the Earth. There are two allotropic forms and five naturally occurring isotopes of this element; Ti-46 through Ti-50 with Ti-48 being the most abundant (73.8%). One of titanium's most notable characteristics is that it is as strong as steel but is only 60% its weight. Titanium's properties are chemically and physically similar to zirconium.
Portal:Chemistry/Featured article/4
Acetic acid (CH3COOH), also known as ethanoic acid, is an organic chemical compound best recognized for giving vinegar its sour taste and pungent smell. Pure water-free acetic acid is a colourless hygroscopic liquid (that is, it readily absorbs water) that freezes below 16.7 °C (62 °F) to a colourless crystalline solid. Acetic acid is corrosive, and its vapour is irritating to eyes and nose, although it is a weak acid based on its ability to dissociate in aqueous solutions.
Acetic acid is one of the simplest carboxylic acids. It is an important chemical reagent and industrial chemical that is used in the production of polyethylene terephthalate mainly in soft drink bottles; cellulose acetate, mainly for photographic film; and polyvinyl acetate for wood glue, as well as many synthetic fibres and fabrics. In households diluted acetic acid is often used in descaling agents. In the food industry acetic acid is used under the food additive code E260 as an acidity regulator.
The global demand for acetic acid is around 6.5 million tonnes per year (Mt/a), of which approximately 1.5 Mt/a is met by recycling; the remainder is manufactured from petrochemical feedstocks or from biological sources.
Portal:Chemistry/Featured article/5
Alchemy is an early protoscientific and philosophical discipline combining elements of chemistry, metallurgy, physics, medicine, astrology, semiotics, mysticism, spiritualism, and art. Alchemy has been practiced in ancient Egypt, India, and China, in Classical Greece and Rome, in the Islamic empire, and then in Europe up to the 19th century — in a complex network of schools and philosophical systems spanning at least 2500 years.
Western alchemy has always been closely connected with Hermeticism, a philosophical and spiritual system that traces its roots to Hermes Trismegistus, a syncretic Egyptian-Greek deity and legendary alchemist. These two disciplines influenced the birth of Rosicrucianism, an important esoteric movement of the 17th century. In the 19th century, as mainstream alchemy evolved into modern chemistry, its mystic and Hermetic aspects became the focus of a modern spiritual alchemy, where material manipulations are viewed as mere symbols of spiritual transformations.
The alchemists did not follow what is now known as the scientific method, and much of the "knowledge" they produced was later found to be banal, limited, wrong, or meaningless. Today, the discipline is of interest mainly to historians of science and philosophy, and for its mystic, esoteric, and artistic aspects. Nevertheless, alchemy was one of the main precursors of modern sciences, and we owe to the ancient alchemists the discovery of many substances and processes that are the mainstay of modern chemical and metallurgical industries.
Portal:Chemistry/Featured article/6
Helium is a chemical element; its atomic symbol is He. It is a colorless, odorless, tasteless, non-toxic, and nearly inert monatomic that heads the noble gas series in the periodic table. Its atomic number is 2 and its boiling and melting points are the lowest among the elements. It exists only as a gas except in extreme conditions. Extreme conditions are also needed to create the small handful of helium compounds, which are all unstable at standard temperature and pressure. Its most abundant stable isotope is helium-4 and it has a rare stable isotope, helium-3. The behavior of liquid helium-4's two different states—helium I and helium II—is important to researchers studying quantum mechanics (in particular the phenomenon of superfluidity) and those looking at the effects that near absolute zero temperatures have on matter (such as superconductivity).
Helium is the second most abundant element in the known Universe and second lightest element in the periodic table. In the modern Universe almost all new helium is created as a result of the nuclear fusion of hydrogen in stars. On Earth it is created by the radioactive decay of much heavier elements (alpha particles are helium-4 nuclei produced by alpha-decay). After its creation, part of it is trapped with natural gas in concentrations up to 7% by volume. It is extracted from the natural gas by a low temperature separation process called fractional distillation.
In 1868 the French astronomer Pierre Janssen first detected helium as an unknown yellow spectral line signature in light from a solar eclipse. (The word helium comes from ancient Greek ἥλιος which is, surprisingly, cognate with the English sun.) Since then large reserves of helium have been found in the natural gas fields of the United States, which is by far the largest supplier of the gas. Helium is used in cryogenics, in deep-sea breathing systems, to cool superconducting magnets, in helium dating, for inflating balloons, for providing lift in airships and as a protective gas for many industrial uses (such as arc welding and growing silicon wafers). Inhaling a small volume of the gas temporarily changes the quality of one's voice.
Portal:Chemistry/Featured article/7
Lysergic acid diethylamide, commonly called LSD, or LSD-25, is a semisynthetic psychedelic drug. The short form LSD comes from "Lysergsäure diethylamid". A typical single dose of LSD during the 1960s was between 100 and 200 micrograms, a tiny amount roughly equal to one-tenth the weight of a grain of sand. Today, a typical single dose of LSD can be as low as 25–50 micrograms, although they are more commonly 50–100 micrograms. Threshold effects can be felt with as little as 20 micrograms.
The effects of LSD can vary greatly, depending on factors such as previous experiences, state of mind and environment, as well as dose strength. Generally, LSD causes expansion and altered experience of senses, emotions, memories, and awareness for 8 to 14 hours. In addition, LSD may produce visual effects such as moving geometric patterns, "trails" behind moving objects, and brilliant colors. LSD does not produce hallucinations in the strict sense but instead illusions and vivid daydream-like fantasies, in which ordinary objects and experiences can take on entirely different appearances or meanings. At higher doses it can cause synaesthesia. The drug sometimes spurs long-term or even permanent changes in a user's personality and life perspective.
LSD is synthesized from lysergic acid derived from ergot, a grain fungus that typically grows on rye. LSD is sensitive to oxygen, ultraviolet light, and chlorine, especially in solution (though its potency may last years if the substance is stored away from light and moisture at low temperature). In pure form it is colorless, odorless, and mildly bitter. LSD is typically delivered orally, usually on a substrate such as absorbent blotter paper, a sugar cube, or gelatin.
Introduced by Sandoz Laboratories as a drug with various psychiatric uses, LSD quickly became a therapeutic agent that appeared to show great promise. However, the extra-medical use of the drug in Western society in the middle years of the twentieth century led to a political firestorm and government insider panic that resulted in the banning of the substance for medical as well as recreational and spiritual uses. Despite this, it is still considered a promising drug in some intellectual circles.
Portal:Chemistry/Featured article/8
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes. More recently it is used as a heterogeneous catalyst in a variety of organic syntheses, most commonly for hydrogenation reactions.
Raney nickel is produced when a block of nickel-aluminium alloy is treated with concentrated sodium hydroxide. This treatment, called "activation", dissolves most of the aluminium out of the alloy. The porous structure left behind has a large surface area, which gives high catalytic activity. A typical catalyst is around 85-percent nickel by mass, corresponding to about two atoms of nickel for every atom of aluminium. The aluminium which remains helps to preserve the pore structure of the overall catalyst.
Since Raney is a registered trademark of W. R. Grace and Company, only those products by its Grace Davison division are properly called "Raney nickel". Alternatively, the more generic terms "skeletal catalyst" or "sponge-metal catalyst" may be used to refer to catalysts that have physical and chemical properties similar to those of Raney nickel.
Portal:Chemistry/Featured article/9
Enzymes are proteins that accelerate, or catalyze, chemical reactions. In these reactions, the molecules at the beginning of the process are called substrates, and the enzyme converts these into different molecules, the products. Almost all processes in the cell need enzymes in order to occur at significant rates. Since enzymes are extremely selective for their substrates and speed up only a few reactions from among many possibilities, the set of enzymes made in a cell determines which metabolic pathways occur in that cell.
Enzymes are known to catalyze about 4,000 reactions. However, not all biological catalysts are proteins, since some RNA molecules called ribozymes can also catalyze reactions. Enzymes are usually named according to the reaction they catalyze. Typically the suffix -ase is added to the name of the substrate (e.g., lactase is the enzyme that cleaves lactose) or the type of reaction (e.g., DNA polymerase forms DNA polymers).
Like all catalysts, enzymes work by providing an alternative path of lower activation energy for a reaction and dramatically accelerating its rate. Some enzymes can make their conversion of substrate to product occur many millions of times faster. For example, the reaction catalysed by orotidine 5'-phosphate decarboxylase will consume half of its substrate in 78 million years if no enzyme is present. However, when the decarboxylase is added, the same process takes just 25 milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. However, enzymes do differ from most other catalysts by being much more specific.
Enzyme activity can be affected by other molecules. Inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Drugs and poisons are often enzyme inhibitors.
Some enzymes are used commercially, for example, in the synthesis of antibiotics. In addition, some household cleaning products use enzymes to speed up chemical reactions (e.g., enzymes in biological washing powders break down protein or fat stains on clothes).
Portal:Chemistry/Featured article/10
Enzyme inhibitors are molecules that bind to enzymes and decrease their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides. However, not all molecules that bind to enzymes are inhibitors; enzyme activators increase enzymatic activity.
The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalysing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically. These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind the enzyme, the enzyme-substrate complex, or both.
Many drug molecules are enzyme inhibitors so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.
Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows flux through a pathway when the products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, such as proteases or nucleases; a well-characterised example is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.
Portal:Chemistry/Featured article/11 Lead(II) nitrate is the inorganic salt of nitric acid and lead. It is colourless crystal or white powder and a strong, stable oxidizer. Unlike most other lead(II) salts, it is soluble in water. Its main use from the Middle Ages under the name plumb dulcis, has been as raw material in the production of many pigments. Since the 20th century, it is industrially used as heat stabilizer in nylon and polyesters, and in coatings of photothermographic paper. Commercial production did not take place until the 19th century in Europe, and in the United States until after 1943, with a typical production process of metallic lead or lead oxide in nitric acid.
Lead(II) nitrate is toxic and probably carcinogenic to humans. It should therefore be handled and stored with the appropriate safety precautions. Ingestion may lead to acute lead poisoning: symptoms include intestinal malfunction, strong abdominal pains, appetite loss, nausea, vomiting and cramps, while longer-term exposure may lead to neurological and renal problems.
When lead(II) nitrate is heated, it decomposes to lead(II) oxide, accompanied by a crackling noise referred to as decrepitation. Due to this property, lead nitrate is sometimes used in pyrotechnics such as fireworks.
Portal:Chemistry/Featured article/12
The cyclol hypothesis was the first structural model of a folded, globular protein. It was developed by Dorothy Wrinch in the late 1930's, and was based on three assumptions. Firstly, the hypothesis assumes that two peptide groups can be crosslinked by a cyclol reaction; these crosslinks are covalent analogs of non-covalent hydrogen bonds between peptide groups. These reactions have been observed in the ergopeptides and other compounds. Secondly, it assumes that, under some conditions, amino acids will naturally make the maximum possible number of cyclol crosslinks, resulting in cyclol molecules and cyclol fabrics. Finally, the hypothesis assumes that globular proteins have a tertiary structure corresponding to Platonic solids and semiregular polyhedra formed of cyclol fabrics with no free edges.
Although incorrect as a model for the structure of globular proteins, several elements of the cyclol model were later verified, such as the cyclol reaction itself and the hypothesis that hydrophobic interactions are chiefly responsible for protein folding. The cyclol hypothesis stimulated many scientists to research questions in protein structure and chemistry, and was a precursor of the more accurate models hypothesized for the DNA double helix and protein secondary structure. The proposal and testing of the cyclol model also provides an excellent illustration of empirical falsifiability acting as part of the scientific method.
Portal:Chemistry/Featured article/13
The aldol reaction is an important carbon-carbon bond formation reaction in organic chemistry. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. Sometimes, the aldol addition product loses a molecule of water during the reaction to form an α,β-unsaturated ketone. This is called an aldol condensation. The aldol reaction was discovered independently by Charles-Adolphe Wurtz and by Alexander Porfyrevich Borodin in 1872. Borodin observed the aldol dimerization of 3-hydroxybutanal from acetaldehyde under acidic conditions. The aldol reaction is used widely in the large scale production of commodity chemicals such as pentaerythritol and in the pharmaceutical industry for the synthesis of optically pure drugs. For example, Pfizer's initial route to the heart disease drug Lipitor (INN: atorvastatin), approved in 1996, employed two aldol reactions, allowing access to multigram-scale quantities of the drug.
Portal:Chemistry/Featured article/14
Caffeine is a xanthine alkaloid compound that acts as a stimulant in humans. Caffeine is sometimes called guaranine when found in guarana, mateine when found in mate, and theine when found in tea. It is found in the leaves and beans of the coffee plant, in tea, yerba mate, and guarana berries, and in small quantities in cocoa, the kola nut and the Yaupon Holly. Overall, caffeine is found in the beans, leaves, and fruit of over 60 plants, where it acts as a natural pesticide that paralyzes and kills certain insects feeding upon them.
Caffeine is a central nervous system (CNS) stimulant, having the effect of temporarily warding off drowsiness and restoring alertness. Beverages containing caffeine, such as coffee, tea, soft drinks and energy drinks enjoy great popularity: caffeine is the world's most widely consumed psychoactive substance. In North America, 90% of adults consume caffeine daily.
Many natural sources of caffeine also contain widely varying mixtures of other xanthine alkaloids, including the cardiac stimulants theophylline and theobromine and other substances such as tannins.
Portal:Chemistry/Featured article/15
Deoxyribonucleic acid (DNA) is a nucleic acid that contains the genetic instructions for the development and function of living organisms. All living things contain DNA, with the exception of some viruses with RNA genomes. The main role of DNA in the cell is the long term storage of information. It is often compared to a blueprint, since it contains the instructions to construct other components of the cell, such as proteins and RNA molecules. The DNA segments that carry genetic information are called genes, but other DNA sequences have structural purposes, or are involved in regulating the expression of genetic information.
DNA is a long polymer of simple units called nucleotides, which are held together by a backbone made of sugars and phosphate groups. This backbone carries four types of molecules called bases and it is the sequence of these four bases that encodes information. The major function of DNA is to encode the sequence of amino acid residues in proteins, using the genetic code. To read the genetic code, cells make a copy of a stretch of DNA in the nucleic acid RNA. These RNA copies can then be used to direct protein synthesis, but they can also be used directly as parts of ribosomes or spliceosomes.
James D. Watson and Francis Crick produced the first accurate model of DNA structure in 1953 in their article The Molecular structure of Nucleic Acids. Watson and Crick proposed the central dogma of molecular biology in 1957, describing how proteins are produced from nucleic DNA. In 1962 Watson, Crick, and Maurice Wilkins jointly received the Nobel Prize.
Portal:Chemistry/Featured article/16
Enzyme kinetics is the study of the rates of chemical reactions that are catalysed by enzymes. The study of an enzyme's kinetics provides insights into the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled in the cell and how drugs and poisons can inhibit its activity.
Enzymes are molecules that manipulate other molecules — the enzymes' substrates. These target molecules bind to an enzyme's active site and are transformed into products through a series of steps known as the enzymatic mechanism. Some enzymes bind multiple substrates and/or release multiple products, such as a protease cleaving one protein substrate into two polypeptide products. Others join substrates together, such as DNA polymerase linking a nucleotide to DNA. Although these mechanisms are often a complex series of steps, there is typically one rate-determining step that determines the overall kinetics. This rate-determining step may be a chemical reaction or a conformational change of the enzyme or substrates, such as those involved in the release of product(s) from the enzyme.
Portal:Chemistry/Featured article/17
Diamond is the hardest known natural material (third-hardest known material after aggregated diamond nanorods and ultrahard fullerite), and is an allotrope of carbon. A diamond is a transparent crystal of tetrahedrally bonded carbon atoms. Diamonds have been adapted for many uses because of the material's exceptional physical characteristics. Most notable are its extreme hardness of diamond, its high dispersion index, and high thermal conductivity.
Portal:Chemistry/Featured article/18
Antioxidants are molecules that slow or prevent the oxidation of other chemicals. Oxidation is a redox chemical reaction that transfers electrons from a substance to an oxidizing agent. Oxidation reactions can involve the production of free radicals, which can form dangerous chain reactions. Antioxidants can terminate these chain reactions by removing radical intermediates and can inhibit other oxidation reactions by being oxidized themselves. As a result, antioxidants are often reducing agents such as thiols or phenols.
Although oxidation reactions are critical for life, they can also be damaging; hence, plants and animals maintain complex systems of multiple types of antioxidants, such as glutathione, vitamin C, and vitamin E as well as enzymes such as catalase, superoxide dismutase and various peroxidases. Low levels of antioxidant molecules or inhibition of these antioxidant enzymes causes oxidative stress and may damage or kill cells.
As oxidative stress has been implicated in the pathogenesis of many human diseases, the use of antioxidants in pharmacology is intensively studied, particularly as treatments for stroke and neurodegenerative diseases. Antioxidants are also widely used as ingredients in dietary supplements in the hope of maintaining health and preventing diseases such as cancer and coronary heart disease. In addition to these uses in medicine, antioxidants have many industrial uses, such as preservatives in food and cosmetics and preventing the degradation of rubber and gasoline.
Portal:Chemistry/Featured article/19
Uranium is a silvery metallic chemical element in the actinide series of the periodic table that has the symbol U and atomic number 92. The heaviest naturally occurring element, uranium is nearly twice as dense as lead and weakly radioactive. It occurs naturally in low concentrations (a few parts per million) in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite (see uranium mining).
In nature, uranium atoms exist as uranium-238 (99.275%), uranium-235 (0.72%), and a very small amount of uranium-234 (0.0058%). Uranium decays slowly by emitting an alpha particle. The half-life of uranium-238 is about 4.5 billion years and that of uranium-235 is 700 million years, making them useful in dating the age of the Earth (see uranium–thorium dating, uranium–lead dating and uranium–uranium dating). Along with thorium and plutonium, it is one of the three fissile elements, meaning it can easily break apart to become lighter elements. This property of uranium-235 and to a lesser degree uranium-233 generates the heat needed to run nuclear reactors and provides the explosive material for nuclear weapons. Both uses rely on the ability of uranium to produce a sustained nuclear chain reaction. Depleted uranium (uranium-238) is used in kinetic energy penetrators and armor plating.
Research by Enrico Fermi and others starting in 1934 led to its use as a fuel in the nuclear power industry and in the first nuclear weapon used in war (see Little Boy and atomic bombings of Hiroshima and Nagasaki). An ensuing arms race during the Cold War between the United States and the Soviet Union produced tens of thousands of nuclear weapons that used enriched uranium and uranium-derived plutonium. The security of those weapons and their fissile material following the dissolution of the Soviet Union in 1991 along with the legacy of nuclear testing and nuclear accidents is a concern for public health and safety.
Portal:Chemistry/Featured article/20
Paracetamol or acetaminophen is a common analgesic and antipyretic drug that is used for the relief of fever, headaches, and other minor aches and pains. Paracetamol is also useful in managing more severe pain, allowing lower dosages of additional non-steroidal anti-inflammatory drugs (NSAIDs) or opioid analgesics to be used, thereby minimizing overall side-effects. It is a major ingredient in numerous cold and flu medications, as well as many prescription analgesics. It is considered safe for human use in recommended doses, but because of its wide availability, deliberate or accidental overdoses are fairly common.
Portal:Chemistry/Featured article/21
Bupropion, previously known as amfebutamone, is an atypical antidepressant that acts as a norepinephrine and dopamine reuptake inhibitor, and nicotinic antagonist. Bupropion belongs to the chemical class of aminoketones and is similar in structure to the stimulant cathinone, to the anorectic diethylpropion, and to phenethylamines in general.
Initially researched and marketed as an antidepressant, bupropion was subsequently found to be effective as a smoking cessation aid. In 2006 it was the fourth-most prescribed antidepressant in the United States retail market, with more than 21 million prescriptions.
Portal:Chemistry/Featured article/22 Francium, formerly known as eka-caesium and actinium K, is a chemical element that has the symbol Fr and atomic number 87. It has the lowest known electronegativity and is the second rarest naturally occurring element (after Astatine). Francium is a highly radioactive metal that decays into astatine, radium, and radon. As an alkali metal, it has one valence electron.
Marguerite Perey discovered francium in 1939. Francium was the last element discovered in nature, rather than synthesized. Outside the laboratory, francium is extremely rare, with trace amounts found in uranium and thorium ores, where the isotope francium-223 is continually formed and continually decays. Perhaps an ounce exists at any given time throughout the Earth's crust; the other isotopes are entirely synthetic. The largest amount ever collected of any isotope was a cluster of 10,000 atoms (of francium-210) created as an ultracold gas at Stony Brook in 1996.
Portal:Chemistry/Featured article/23
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate (ATP). Although the many forms of life on Earth utilize a range of different nutrients, almost all carry out oxidative phosphorylation to produce ATP, the molecule that supplies energy to metabolism. This pathway is probably so pervasive because it is a highly efficient way of storing energy, compared to alternative fermentation processes such as glycolysis.
Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as free radicals that damage cells and contribute to ageing and disease. The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.
Nominations
[edit]Feel free to add featured or top/high importance articles to the list above. Other articles may be nominated here.