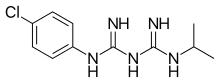
Proguanil
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Paludrine, others |
| Other names | chlorguanide, chloroguanide[1] |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 75% |
| Metabolism | By liver (CYP2C19) |
| Metabolites | cycloguanil and 4-chlorophenylbiguanide |
| Elimination half-life | 12–21 hours[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.196 |
| Chemical and physical data | |
| Formula | C11H16ClN5 |
| Molar mass | 253.73 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 129 °C (264 °F) |
| |
| |
| (verify) | |
Proguanil, also known as chlorguanide and chloroguanide, is a medication used to treat and prevent malaria.[3][4] It is often used together with chloroquine or atovaquone.[4][3] When used with chloroquine the combination will treat mild chloroquine resistant malaria.[3] It is taken by mouth.[4]
Side effects include diarrhea, constipation, skin rashes, hair loss, and itchiness.[3] Because malaria tends to be more severe in pregnancy, the benefit typically outweighs the risk.[3] If used during pregnancy it should be taken with folate.[3] It is likely safe for use during breastfeeding.[3] Proguanil is converted by the liver to its active metabolite, cycloguanil.[4]
It is on the World Health Organization's List of Essential Medicines.[5] In the United States and Canada it is only available in combination as atovaquone/proguanil.[6]
History
[edit]When the Japanese attack on Pearl Harbor started World War II in the Pacific, the US became very interested in antimalarial medications and funded a large joint US-UK program to find new non-toxic and easy to produce drugs of the type.[7] It was joined by a team led by Frank Rose at the Medical Chemicals Section of Imperial Chemical Industries (later Pharmaceuticals Division, which ended up demerged into Zeneca) at Blackley, which earlier developed a way to manufacture mepacrine, an antimalarial made exclusively in Germany before the war.[7]
Rose and his colleague Frank Curd decided to concentrate on pyrimidines as relatively simple to synthetise, even though the Advisory Panel recommended against that because most antimalarials by then were either quinolines or acridines. Checking prospective 2,4-diaminopyridine derivatives with a basic side chain and a benzenoid moiety one after another, they noticed a geometric pattern in the effective analogs and wondered if they could reproduce their interesting biologic activity with molecules even simpler, without the pyrimidine ring, and tried biguanides (then called diguanides) with which Rose was familiar due to his earlier sulphonamide research to great effect.[8] The drug was introduced by ICI in 1945.
Medical uses
[edit]Proguanil is used for the prevention and treatment of malaria in both adults and children, particularly in areas where chloroquine-resistant P. falciparum malaria has been reported. It is usually taken in combination with atovaquone, another antimalarial drug.[9]
It is also effective in the treatment of most other multi-drug resistant forms of P. falciparum; the success rate exceeds 93%.[10]
Side effects
[edit]Proguanil is generally well tolerated, and most people do not experience side effects. However, common side effects include abdominal pain, nausea, headache, and fever. Taking proguanil with food may lessen these side effects.[11] Proguanil should not be taken by people with severe renal impairment, pregnant women, or women who are breastfeeding children less than 5 kg.[12] There have also been reports of increased levels of liver enzymes, which may remain high for up to 4 weeks after completion of treatment.[13]
Mechanism
[edit]When used alone, proguanil functions as a prodrug. Its active metabolite, cycloguanil, is an inhibitor of dihydrofolate reductase (DHFR).[14] Although both mammals and parasites produce DHFR, cycloguanil's inhibitory activity is specific for parasitic DHFR. This enzyme is a critical component of the folic acid cycle. Inhibition of DHFR prevents the parasite from recycling dihydrofolate back to tetrahydrofolate (THF). THF is required for DNA synthesis, amino acid synthesis, and methylation; thus, DHFR inhibition shuts down these processes.[15]
Proguanil displays synergism when used in combination with the antimalarial atovaquone. This mechanism of action differs from when proguanil was used as a singular agent. In this case, it is not thought to function as a DHFR inhibitor. The addition of proguanil has shown to reduce resistance to atovaquone and increase the ability of atovaquone to trigger a mitochondrial apoptotic cascade.[16] This is commonly referred to as "collapse of the mitochondrial membrane potential."[17] Proguanil lowers the effective concentration of atovaquone needed to increase permeability of the mitochondrial membrane.[18]
References
[edit]- ^ Mehlhorn H (2008). "Proguanil". Encyclopedia of Parasitology: A-M. Springer Science & Business Media. p. 388. ISBN 9783540489948. Archived from the original on 20 December 2016.
- ^ "Malarone (atovaquone/proguanil) Tablets, Pediatric Tablets. Full Prescribing Information" (PDF). GlaxoSmithKline. Research Triangle Park, NC 27709. Archived (PDF) from the original on 20 September 2016. Retrieved 14 July 2016.
- ^ a b c d e f g World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 199, 203. hdl:10665/44053. ISBN 9789241547659.
- ^ a b c d "Atovaquone and Proguanil Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Proguanil". www.medscape.com. Medscape. Archived from the original on 9 November 2016. Retrieved 8 November 2016.
- ^ a b Quirke VM (2004). "War and Change in the Pharmaceutical Industry: A Comparative Study of Britain and France in the Twentieth Century". Entreprises et Histoire. 36 (2): 64. doi:10.3917/eh.036.0064.
- ^ Suckling CW, Langley BW (1990). "Francis Leslie Rose: 27 June 1909-3 March 1988". Biographical Memoirs of Fellows of the Royal Society. 36: 491–524 (503-505). doi:10.1098/rsbm.1990.0041. PMID 11616178. S2CID 40302178.
- ^ "Malaria". MedlinePlus Medical Encyclopedia. U.S. National Library of Medicine. Archived from the original on 17 November 2016. Retrieved 16 November 2016.
- ^ Boggild AK, Parise ME, Lewis LS, Kain KC (February 2007). "Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II)". The American Journal of Tropical Medicine and Hygiene. 76 (2): 208–223. doi:10.4269/ajtmh.2007.76.208. PMID 17297027.
- ^ "Atovaquone And Proguanil (Oral Route) Side Effects". Mayo Clinic. Archived from the original on 9 November 2016. Retrieved 8 November 2016.
- ^ "CDC - Malaria - Travelers - Choosing a Drug to Prevent Malaria". U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 13 November 2016. Retrieved 8 November 2016.
- ^ Looareesuwan S, Wilairatana P, Chalermarut K, Rattanapong Y, Canfield CJ, Hutchinson DB (April 1999). "Efficacy and safety of atovaquone/proguanil compared with mefloquine for treatment of acute Plasmodium falciparum malaria in Thailand". The American Journal of Tropical Medicine and Hygiene. 60 (4): 526–532. doi:10.4269/ajtmh.1999.60.526. PMID 10348224. S2CID 32365159.
- ^ "Proguanil | C11H16ClN5". PubChem. U.S. National Library of Medine. Archived from the original on 14 November 2016. Retrieved 13 November 2016.
- ^ Boggild AK, Parise ME, Lewis LS, Kain KC (February 2007). "Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II)". The American Journal of Tropical Medicine and Hygiene. 76 (2): 208–223. doi:10.4269/ajtmh.2007.76.208. PMID 17297027.
- ^ Srivastava IK, Vaidya AB (June 1999). "A mechanism for the synergistic antimalarial action of atovaquone and proguanil". Antimicrobial Agents and Chemotherapy. 43 (6): 1334–1339. doi:10.1128/AAC.43.6.1334. PMC 89274. PMID 10348748.
- ^ Srivastava IK, Rottenberg H, Vaidya AB (February 1997). "Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite". The Journal of Biological Chemistry. 272 (7): 3961–3966. doi:10.1074/jbc.272.7.3961. PMID 9020100.
- ^ Thapar MM, Gupta S, Spindler C, Wernsdorfer WH, Björkman A (May 2003). "Pharmacodynamic interactions among atovaquone, proguanil and cycloguanil against Plasmodium falciparum in vitro". Transactions of the Royal Society of Tropical Medicine and Hygiene. 97 (3): 331–337. doi:10.1016/S0035-9203(03)90162-3. PMID 15228254.
