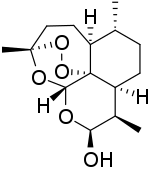
Dihydroartemisinin
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 12% |
| Metabolism | Liver |
| Elimination half-life | About 4–11 hours |
| Excretion | Mainly bile |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.128.242 |
| Chemical and physical data | |
| Formula | C15H24O5 |
| Molar mass | 284.352 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dihydroartemisinin (also known as dihydroqinghaosu, artenimol or DHA) is a drug used to treat malaria. Dihydroartemisinin is the active metabolite of all artemisinin compounds (artemisinin, artesunate, artemether, etc.) and is also available as a drug in itself. It is a semi-synthetic derivative of artemisinin and is widely used as an intermediate in the preparation of other artemisinin-derived antimalarial drugs.[1] It is sold commercially in combination with piperaquine and has been shown to be equivalent to artemether/lumefantrine.[2]
Medical use
[edit]Dihydroartemisinin is used to treat malaria, generally as a combination drug with piperaquine.[3]
In a systematic review of randomized controlled trials, both dihydroartemisinin-piperaquine and artemether-lumefantrine are very effective at treating malaria (high quality evidence). However, dihydroartemisinin-piperaquine cures slightly more patients than artemether-lumefantrine, and it also prevents further malaria infections for longer after treatment (high quality evidence). Dihydroartemisinin-piperaquine and artemether-lumefantrine probably have similar side effects (moderate quality evidence). The studies were all conducted in Africa. In studies of people living in Asia, dihydroartemisinin-piperaquine is as effective as artesunate plus mefloquine at treating malaria (moderate quality evidence). Artesunate plus mefloquine probably causes more nausea, vomiting, dizziness, sleeplessness, and palpitations than dihydroartemisinin-piperaquine (moderate quality evidence).[4]
Pharmacology and mechanism
[edit]
The proposed mechanism of action of artemisinin involves cleavage of endoperoxide bridges by iron, producing free radicals (hypervalent iron-oxo species, epoxides, aldehydes, and dicarbonyl compounds) which damage biological macromolecules causing oxidative stress in the cells of the parasite.[5] Malaria is caused by apicomplexans, primarily Plasmodium falciparum, which largely reside in red blood cells and itself contains iron-rich heme-groups (in the form of hemozoin).[6] In 2015 artemisinin was shown to bind to a large number targets suggesting that it acts in a promiscuous manner.[7] Recent mechanism research discovered that artemisinin targets a broad spectrum of proteins in the human cancer cell proteome through heme-activated radical alkylation.[7]
Chemistry
[edit]Dihydroartemisinin has a low solubility in water of less than 0.1 g/L. Consequently, its use may result in side effects caused by minor, yet much more soluble, additives (excipients) such as Cremophor EL.[8]
The lactone of artemisinin could selectively be reduced with mild hydride-reducing agents, such as sodium borohydride, potassium borohydride, and lithium borohydride to dihydroartemisinin (a lactol) in over 90% yield. It is a novel reduction, because normally lactones cannot be reduced with sodium borohydride under the same reaction conditions (0–5 ˚C in methanol). Reduction with LiAlH4 leads to some rearranged products. It was surprising to find that the lactone was reduced, but that the peroxy group survived. However, the lactone of deoxyartemisinin resisted reduction with sodium borohydride and could only be reduced with diisobutylaluminium hydride to the lactol deoxydihydroartimisinin. These results show that the peroxy group assists the reduction of lactone with sodium borohydride to a lactol, but not to the alcohol which is the over-reduction product. No clear evidence for this reduction process exists.[citation needed]
Society and culture
[edit]In combination with piperaquine, brands include:[citation needed]
- D-Artepp (GPSC)
- Artekin (Holleykin)
- Diphos (Genix Pharma)
- TimeQuin (Sami Pharma)
- Eurartesim (Sigma Tau; by Good Manufacturing Practices)
- Duocotecxin (Holley Pharm)
Alone:[citation needed]
- Cotecxin (Zhejiang Holley Nanhu Pharmaceutical Co.)
Research
[edit]Accumulative research suggests that dihydroartemisinin and other artemisinin-based endoperoxide compounds may display activity as experimental cancer chemotherapeutics.[9] Recent pharmacological evidence demonstrates that dihydroartemisinin targets human metastatic melanoma cells with induction of NOXA-dependent mitochondrial apoptosis that occurs downstream of iron-dependent generation of cytotoxic oxidative stress.[10]
References
[edit]- ^ Woo SH, Parker MH, Ploypradith P, Northrop J, Posner GH (1998). "Direct conversion of pyranose anomeric OH→F→R in the artemisinin family of antimalarial trioxanes". Tetrahedron Letters. 39 (12): 1533–6. doi:10.1016/S0040-4039(98)00132-4.
- ^ Arinaitwe E, Sandison TG, Wanzira H, Kakuru A, Homsy J, Kalamya J, et al. (December 2009). "Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in young Ugandan children". Clinical Infectious Diseases. 49 (11): 1629–1637. doi:10.1086/647946. PMID 19877969.
- ^ Tilley L, Straimer J, Gnädig NF, Ralph SA, Fidock DA (September 2016). "Artemisinin Action and Resistance in Plasmodium falciparum". Trends in Parasitology. 32 (9): 682–696. doi:10.1016/j.pt.2016.05.010. PMC 5007624. PMID 27289273.
- ^ Zani B, Gathu M, Donegan S, Olliaro PL, Sinclair D (January 2014). "Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria". The Cochrane Database of Systematic Reviews. 2014 (1): CD010927. doi:10.1002/14651858.CD010927. PMC 4470355. PMID 24443033.
- ^ Cumming JN, Ploypradith P, Posner GH (1996). Antimalarial activity of artemisinin (Qinghaosu) and related trioxanes: Mechanism(s) of action. Advances in Pharmacology. Vol. 37. pp. 253–297. doi:10.1016/S1054-3589(08)60952-7. ISBN 9780120329380. PMID 8891104.
- ^ Posner GH, O'Neill PM (June 2004). "Knowledge of the proposed chemical mechanism of action and cytochrome p450 metabolism of antimalarial trioxanes like artemisinin allows rational design of new antimalarial peroxides". Accounts of Chemical Research. 37 (6): 397–404. doi:10.1021/ar020227u. PMID 15196049.
- ^ a b Zhou Y, Li W, Xiao Y (April 2016). "Profiling of Multiple Targets of Artemisinin Activated by Hemin in Cancer Cell Proteome". ACS Chemical Biology. 11 (4): 882–888. doi:10.1021/acschembio.5b01043. PMID 26854499.
- ^ Liu K, Dai L, Li C, Liu J, Wang L, Lei J (July 2016). "Self-assembled targeted nanoparticles based on transferrin-modified eight-arm-polyethylene glycol-dihydroartemisinin conjugate". Scientific Reports. 6: 29461. Bibcode:2016NatSR...629461L. doi:10.1038/srep29461. PMC 4932499. PMID 27377918.
- ^ Efferth T (April 2006). "Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells". Current Drug Targets. 7 (4): 407–421. doi:10.2174/138945006776359412. PMID 16611029.
- ^ Cabello CM, Lamore SD, Bair WB, Qiao S, Azimian S, Lesson JL, et al. (August 2012). "The redox antimalarial dihydroartemisinin targets human metastatic melanoma cells but not primary melanocytes with induction of NOXA-dependent apoptosis". Investigational New Drugs. 30 (4): 1289–1301. doi:10.1007/s10637-011-9676-7. PMC 3203350. PMID 21547369.
Further reading
[edit]- Jansen FH (July 2010). "The pharmaceutical death-ride of dihydroartemisinin". Malaria Journal. 9: 212. doi:10.1186/1475-2875-9-212. PMC 2916014. PMID 20649950.
