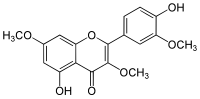
Pachypodol
Appearance
| |
| |
| Names | |
|---|---|
| IUPAC name
4′,5-Dihydroxy-3,3′,7-trimethoxyflavone
| |
| Systematic IUPAC name
5-Hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7-dimethoxy-4H-1-benzopyran-4-one | |
| Other names
Quercetin 3,7,3'-trimethyl ether
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | C008751 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H16O7 | |
| Molar mass | 344.319 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pachypodol is a chemical compound classified as an O-methylated flavonol. It can be isolated from a variety of plants including Calycopteris floribunda,[1] Pogostemon cablin,[2] and Croton ciliatoglanduliferus.[3]
References
[edit]- ^ Ali, Husne-Ara; Chowdhury, A. K. Azad; Rahman, Abul K. M.; Borkowski, Tomasz; Nahar, Lutfun; Sarker, Satyajit D. (2008). "Pachypodol, a flavonol from the leaves of Calycopteris floribunda, inhibits the growth of CaCo 2 colon cancer cell line in vitro". Phytotherapy Research. 22 (12): 1684–1687. doi:10.1002/ptr.2539. PMID 18570232. S2CID 1498024.
- ^ Umar Ijaz, Muhammad; Rauf, Ayesha; Mustafa, Shama; Ahmed, Hussain; Ashraf, Asma; Al-Ghanim, Khalid; Swamy Mruthinti, Satyanarayana; Mahboob, S. (2022). "Pachypodol attenuates Perfluorooctane sulphonate-induced testicular damage by reducing oxidative stress". Saudi Journal of Biological Sciences. 29 (3): 1380–1385. doi:10.1016/j.sjbs.2021.12.012. PMC 8913419. PMID 35280584.
- ^ González-Vázquez, Raquel; King Díaz, Beatriz; Aguilar, María Isabel; Diego, Nelly; Lotina-Hennsen, Blas (2006). "Pachypodol from Croton ciliatoglanduliferus Ort. As Water-Splitting Enzyme Inhibitor on Thylakoids". Journal of Agricultural and Food Chemistry. 54 (4): 1217–1221. doi:10.1021/jf051897s. PMID 16478239.
