Niche (protein structural motif)
In the area of protein structural motifs, niches are three or four amino acid residue features in which main-chain CO groups are bridged by positively charged or δ+ groups.[1][2][3] The δ+ groups include groups with two hydrogen bond donor atoms such as NH2 groups and water molecules. In typical proteins, 7% of amino acid residues belong to niches bound to a δ+ group, while another 7% have the conformation but no single cationic bridging group is detected.
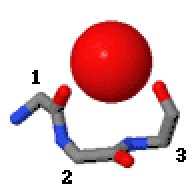
Niches are of two kinds, distinguished as niche3 (3 residues, i to i+2) and niche4 (4 residues, i to i+3). In a niche3 motif the δ+-binding carbonyl groups are from residues i and i+2 while in a niche4 motif they are from residues i and i+3.
A niche3 has the α conformation for residue i+1 and the β conformation for residue i+2; a niche4 has the α conformation for residues i+1 and i+2 and the β conformation for residue i+3.
A niche occurs commonly at the C-terminus of α-helices and especially of 310 helices.
Metal ions that occur bound to niches in proteins are Na+, K+, Ca2+, and Mg2+. Proteins with regulatory cations often employ niches for metal binding (e.g., thrombin, Na+; annexin, Ca2+; pyruvate dehydrogenase, K+).
A major cation transporter in cells is calcium ATPase.[4] In the Ca2+-bound crystal structures the two calcium ions side-by-side within the transmembrane domain are thought to be at the halfway stage of being transported. As well as being bound by various side chain carbonyl groups, one of these calcium ions is bound by a niche3/niche4 (both in the one motif) at residues 304–307 at the C-terminus of an α-helix.
A lysine side chain in the nuclear export receptor CRM1 is recognised specifically by a niche conformation that has to be adopted as a key part of the nuclear export signal of proteins exiting the nucleus.[5]
A sodium ion in the Fluc fluoride channel is situated at the dyad axis of the dimer, bound tetrahedrally by two niche4s, one from each subunit.[6] A Sodium ion bound in similar ways at the domain interface is seen in several other Na+-coupled transporters.[7]
The Hsp70 interdomain linker region of 10 residues enables allosteric communication between two folded domains. The N-terminal part of the linker has a niche4 structure that is water-bound.[8]
In the scorpion toxin BeM9 the side chain of arginine 60 binds the carbonyls of residues 61 and 63 as a niche3. The motif, loss of which alters the specificity of the protein for voltage-gated sodium channels, is named "arginine hand".[9] The slightly unusual dihedral angles for a niche3 are because this niche3 accommodates two separate NH groups from the arginine's guanidino group.
Another small tripeptide motif that binds cations or δ+ groups via main-chain CO groups is called the catgrip.
References
[edit]- ^ Torrance, GM; Leader DP (2009). "A Novel Main Chain Motif in Proteins Bridged by Cationic Groups: The Niche". Journal of Molecular Biology. 385 (4): 1076–1086. doi:10.1016/j.jmb.2008.11.007. PMID 19038265.
- ^ Regad, L; Martin J (2011). "Dissecting protein loops with a statistical scalpel suggests a functional implication of some structural motifs". BMC Bioinformatics. 12 (1): 247. doi:10.1186/1471-2105-12-247. PMC 3158783. PMID 21689388.
- ^ Cianci, M; Tomaszewski (2010). "Crystallographic Analysis of Counterion Effects on Subtilisin Enzymatic Action in Acetonitrile". Journal of the American Chemical Society. 132 (7): 2293–2300. doi:10.1021/ja908703c. PMID 20099851.
- ^ Toyoshima, C; Mizutani (2004). "Crystal structure of the calcium pump with a bound ATP analogue". Nature. 430 (6999): 529–535. doi:10.1038/nature02680. PMID 15229613. S2CID 4331138.
- ^ Fung, HYJ; Fu S-C; Chook YM (2017). "Nuclear export receptor CRM1 recognizes diverse conformations in nuclear export signals". eLife. 6: e23961. doi:10.7554/eLife.23961. PMC 5358978. PMID 28282025.
- ^ Stockbridge, RB; Kolmakova-Partensky L; Shane T (2015). "Crystal structures of a double-barrelled fluoride ion channel". Nature. 525 (7570): 548–551. doi:10.1038/nature14981. PMC 4876929. PMID 26344196.
- ^ McIlwain, BC; Martin K (2020). "An Interfacial Sodium Ion is an Essential Structural Feature of Fluc Family Fluoride Channels". Journal of Molecular Biology. 432 (4): 1098–1108. doi:10.1016/j.jmb.2020.01.007. PMC 7054162. PMID 31945374.
- ^ English, CA; Sherman W; Meng W (2017). "The Hsp70 interdomain linker is a dynamic switch that enables allosteric communication between two structured domains". J Biol Chem. 292 (36): 14765–14774. doi:10.1074/jbc.M117.789313. PMC 5592658. PMID 28754691.
- ^ Kuldyushev, NA; Mineev KS; Berkut AA (2018). "Refined structure of BeM9 reveals arginine hand, a motif in scorpion toxins affecting sodium channels". Proteins. 86 (10): 1117–1122. doi:10.1002/prot.25583. PMID 30007037. S2CID 51625592.
External links
[edit]- Motivated Proteins: [1]; Leader, DP; Milner-White (2009). "Motivated Proteins: A web application for studying small three-dimensional protein motifs". BMC Bioinformatics. 10 (1): 60. doi:10.1186/1471-2105-10-60. PMC 2651126. PMID 19210785.
- PDBeMotif: [2]; Golovin, A; Henrick (2008). "MSDmotif: exploring protein sites and motifs". BMC Bioinformatics. 9 (1): 312. doi:10.1186/1471-2105-9-312. PMC 2491636. PMID 18637174.
