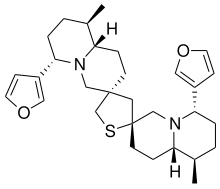
Neothiobinupharidine
Appearance
This article needs additional citations for verification. (May 2022) |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2′R,3′′R,6S,6′′S,9R,9′′R,9aS,9′′aS)-6,6′′-Di(furan-3-yl)-9,9′′-dimethyldodecahydro-2H,2′′H,4H,4′′H-dispiro[quinolizine-3,2′-thiolane-4′,3′′-quinolizine] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H42N2O2S | |
| Molar mass | 494.74 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Neothiobinupharidine is a dimeric thiaspirane alkaloid isolated from the dwarf water lily Nuphar pumila. It exhibits weak immunosuppressive and cytotoxic bioactivity in cell line experiments.[1]
References
[edit]- ^ Matsuda, Hisashi; Nakamura, Seikou; Nakashima, Souichi; Fukaya, Masashi; Yoshikawa, Masayuki (2019). "Biofunctional Effects of Thiohemiaminal-Type Dimeric Sesquiterpene Alkaloids from Nuphar Plants". Chem. Pharm. Bull. 67 (7): 666–674. doi:10.1248/cpb.c18-01030. PMID 31257322. S2CID 195758107.
