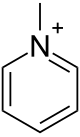
Methylpyridinium
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Methylpyridin-1-ium | |
| Other names
N-Methylpyridinium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H8N+ | |
| Molar mass | 94.134 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methylpyridinium is an ion with the formula C5H5NCH+3. It is the N-methylated derivative of pyridine. It confers no color to its salts. The ion is classified as an quaternary ammonium ion.[1]
Preparation and occurrence
[edit]Methylpyridinium is prepared by treating pyridine with dimethylsulfate:[2]
- C5H5N + (CH3O)2SO2 → [C5H5NCH3]+CH3OSO−3
It is found in some coffee products.[3] It is not present in unroasted coffee beans, but is formed during roasting from its precursor chemical, trigonelline.[3] It is under investigation by scientists regarding its potential anti-carcinogenic properties,[4] particularly an effect on colon cancer.[3]
Ionic liquid
[edit]The chloride salt of N-methylpyridinium behaves as an ionic liquid. Mixtures of that salt and zinc chloride have been characterised over the temperature range 150–200 °C (423–473 K).[5][6][7][8]
See also
[edit]References
[edit]- ^ Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2000). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. p. 558. doi:10.1002/14356007.a22_399. ISBN 9783527303854.
- ^ E. A. Prill, S. M. McElvain (1935). "1-Methyl-2-Pyridone". Organic Syntheses. 15: 41. doi:10.15227/orgsyn.015.0041.
- ^ a b c "Highly Active Compound Found In Coffee May Prevent Colon Cancer". ScienceDaily. Oct 15, 2003. Retrieved Oct 10, 2012.
- ^ Boettler, U; Volz, N; Pahlke, G; Teller, N; Kotyczka, C; Somoza, V; Stiebitz, H; Bytof, G; et al. (2011). "Coffees rich in chlorogenic acid or N-methylpyridinium induce chemopreventive phase II-enzymes via the Nrf2/ARE pathway in vitro and in vivo". Molecular Nutrition & Food Research. 55 (5): 798–802. doi:10.1002/mnfr.201100115. PMID 21448860.
- ^ Simonis, L.; Coppe, C.; Glibert, J.; Claes, P. (1986). "Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – I. Phase diagram and heats of mixing". Thermochimica Acta. 99: 223–232. Bibcode:1986TcAc...99..223S. doi:10.1016/0040-6031(86)85285-6.
- ^ Claes, P.; Simonis, L.; Glibert, J. (1986). "Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – II. Specific mass, electrical conductivity and viscosity". Electrochimica Acta. 31 (12): 1525–1530. doi:10.1016/0013-4686(86)87071-2.
- ^ Claes, P. F.; Coppe, C. R.; Simonis, L. A.; Glibert, J. E. (1987). "Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – III. Solubility of hydrogen chloride under atmospheric pressure and comparison with zinc chloride—N-ethylpyridinium bromide mixtures". Journal of Chemical and Engineering Data. 32 (1): 70–72. doi:10.1021/je00047a020.
- ^ Marković, R.; Minić, D. M. (1997). "Conductometric and thermal studies of fused Zn(II) salts containing methyl substituted pyridinium cations". Materials Chemistry and Physics. 50 (1): 20–24. doi:10.1016/S0254-0584(97)80178-2.
