Metallodendrimer
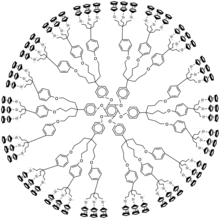
A metallodendrimer is a type of dendrimer with incorporated metal atoms. The development of this type of material is actively pursued in academia.[1][2][3]
Structure
[edit]The metal can be situated in the repeat unit, the core or at the extremities as end-group. Elements often encountered are palladium and platinum. These metals can form octahedral six-coordinate M(IV) linking units from organic dihalides and the corresponding 4-coordinate M(II) monomers. Ferrocene-containing dendrimers and dendrimers with cobaltocene and arylchromiumtricarbonyl units have been reported in end-functional dendrimers.
Metallodendrimers can form as metal complexes with dendritic counter ions for example by hydrolysis of ester terminated PAMAM dendrimers with sodium hydroxide.
Applications
[edit]Metallodendrimers are investigated as equivalents to nanoparticles. Applications can be expected in the fields of catalysis, as chemical sensors in molecular recognition - for example of bromine and chloride anions [4] - or as materials capable of binding metals. Metallodendrimers can also mimic certain biomolecules for example haemoprotein in dendrimer with a porphyrin core. Further uses are reported as electrocatalyst.[5][6]
Examples of metallodendrimer heterogeneous catalysis are a nickel-containing dendrimer active in the Kharasch addition,[7] palladium-containing dendrimers active in ethylene polymerization[8] and in the Heck reaction.[9]
References
[edit]- ^ Gorman, C. (1998). "Metallodendrimers: Structural Diversity and Functional Behavior". Advanced Materials. 10 (4): 295–309. doi:10.1002/(SICI)1521-4095(199803)10:4<295::AID-ADMA295>3.0.CO;2-N.
- ^ Cuadrado, I.; Morán, M. S.; Casado, C. M.; Alonso, B.; Losada, J. (1999). "Organometallic dendrimers with transition metals". Coordination Chemistry Reviews. 193–195: 395–445. doi:10.1016/S0010-8545(99)00036-3.
- ^ Stoddart, F.; Welton, T. (1999). "Metal-containing dendritic polymers". Polyhedron. 18 (27): 3575. doi:10.1016/S0277-5387(99)00301-0. hdl:10044/1/9945.
- ^ Valério, C.; Alonso, E.; Ruiz, J.; Blais, J. C.; Astruc, D. (1999). "A Polycationic Metallodendrimer with 24 [Fe(η5-C5Me5)(η6-N-Alkylaniline)]+ Termini That Recognizes Chloride and Bromide Anions". Angewandte Chemie International Edition. 38 (12): 1747. doi:10.1002/(SICI)1521-3773(19990614)38:12<1747::AID-ANIE1747>3.0.CO;2-G.
- ^ Cheng, L.; Cox, J. A. (2002). "Nanocomposite Multilayer Film of a Ruthenium Metallodendrimer and a Dawson-Type Polyoxometalate as a Bifunctional Electrocatalyst". Chemistry of Materials. 14: 6–8. doi:10.1021/cm010854y.
- ^ Cheng, L.; Pacey, G. E.; Cox, J. A. (2001). "Carbon Electrodes Modified with Ruthenium Metallodendrimer Multilayers for the Mediated Oxidation of Methionine and Insulin at Physiological pH". Analytical Chemistry. 73 (22): 5607–5610. doi:10.1021/ac0105585. PMID 11816594.
- ^ Knapen, J. W. J.; Van Der Made, A. W.; De Wilde, J. C.; Van Leeuwen, P. W. N. M.; Wijkens, P.; Grove, D. M.; Van Koten, G. (1994). "Homogeneous catalysts based on silane dendrimers functionalized with arylnickel(II) complexes". Nature. 372 (6507): 659. Bibcode:1994Natur.372..659K. doi:10.1038/372659a0. hdl:1874/6274.
- ^ Smith, G.; Chen, R.; Mapolie, S. (2003). "The synthesis and catalytic activity of a first-generation poly(propylene imine) pyridylimine palladium metallodendrimer". Journal of Organometallic Chemistry. 673 (1–2): 111–035. doi:10.1016/S0022-328X(03)00173-6.
- ^ Smith, G.; Mapolie, S. F. (2004). "Iminopyridyl-palladium dendritic catalyst precursors: evaluation in Heck reactions". Journal of Molecular Catalysis A: Chemical. 213 (2): 187–192. doi:10.1016/j.molcata.2003.12.010.
