Metal salen complex
A metal salen complex is a coordination compound between a metal cation and a ligand derived from N,N′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent cobalt Co2+, usually denoted as Co(salen).[1] These complexes are widely investigated as catalysts and enzyme mimics.[2][3]

The metal-free salen compound (H2salen or salenH2) has two phenolic hydroxyl groups. The salen ligand is usually its conjugate base (salen2−), resulting from the loss of protons from those hydroxyl groups. The metal atom usually makes four coordination bonds to the oxygen and nitrogen atoms.
Preparation of complexes
[edit]The salen anion forms complexes with most transition metals. These complexes are usually prepared by the reaction of H2salen ("proligand") with metal precursors containing built-in bases, such as alkoxides, metal amides, or metal acetate. The proligand may also be treated with a metal halide, with or without an added base. Lastly, the proligand may be deprotonated by a nonnucleophilic base, such as sodium hydride, before treatment with the metal halide. For example, Jacobsen's catalyst is prepared from the salen ligand precursor with manganese acetate.[4]
Structures
[edit]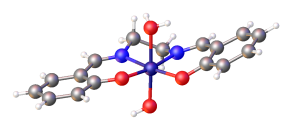
Salen complexes with d8 metal ions, such as Ni(salen), typically have a low-spin square planar molecular geometry in the coordination sphere.
Other metal–salen complexes may have additional ligands above the salen nitrogen–oxygen plane. Complexes with one extra ligand, such as VO(salen),[6] may have a square pyramidal molecular geometry. Complexes with two extra ligands, such as Co(salen)Cl(py), may have octahedral geometry. Usually the MN2O2 core is relatively planar, even though the ethylene backbone is skewed and the overall salen ligand takes a twisted C2 symmetry. Examples exist where ancillary ligands force the N2O2 donors out of planarity.[7] No evidence indicates that salen is a redox-noninnocent ligand.
Reactions
[edit]
The pyridine adduct of the cobalt(II) complex Co(salen)(py) (salcomine) has a square-pyramidal structure. It is a dioxygen carrier by forming a labile, octahedral O2 complex.[9][10]
The name "salen ligands" is used for tetradentate ligands which have similar structures. For example, in salpn there is a methyl substituent on the bridge. It is used as a metal deactivation additive in fuels.[11] The presence of bulky groups near the coordination site may enhance the catalytic activity of a metal complex and prevent its dimerization. Salen ligands derived from 3,5-di-tert-butylsalicylaldehyde fulfill these roles, and also increase the solubility of the complexes in non-polar solvents like pentane. Chiral "salen" ligands may be created by proper substitution of the diamine backbone, the phenyl ring, or both.[12] An example is the ligand obtained by condensation of the C2-symmetric trans-1,2-diaminocyclohexane with 3,5-di-tert-butylsalicylaldehyde. Chiral ligands may be used in asymmetric synthesis reactions, such as the Jacobsen epoxidation:[4][13]
History
[edit]Tsumaki described the first metal–salen complexes in 1938. He found that the cobalt(II) complex Co(salen) reversibly binds O2, which led to intensive research on cobalt complexes of salen and related ligands for their capacity for oxygen storage and transport, looking for potential synthetic oxygen carriers.[1] Cobalt salen complexes also replicate certain aspects of vitamin B12.
The manganese-containing salen complex catalyzes the asymmetric epoxidation of alkenes. In the hydrolytic kinetic resolution technique, a racemic mixture of epoxides may be separated by selectively hydrolyzing one enantiomer, catalyzed by the analogous cobalt(III) complex.[14] In subsequent work, chromium(III) and cobalt(III) salen complexes catalyze the reaction of carbon dioxide and epoxides to give polycarbonates.[15]
Related complexes
[edit]Substituted salen complexes
[edit]

Complexes of salen per se are poorly soluble in organic solvents. Substitution of the organic framework increases the solubility of the complex. An example is the salpn ligand, derived from 1,2-diaminopropane instead of ethylenediamine, which is used as a metal deactivating additive in motor oils and motor fuel.[16]
The presence of bulky groups adjacent to the phenoxide group can give complexes with enhanced catalytic activity. These substituents suppress formation of dimers. For these reasons, salen ligands derived from 3,5-di-tert-butylsalicylaldehyde have received particular scrutiny.
Chirality may be introduced into the ligand either via the diamine backbone, via the phenyl ring, or both.[12] For example, condensation of the C2-symmetric trans-1,2-diaminocyclohexane with 3,5-di-tert-butylsalicylaldehdye gives a ligand that forms complexes with Cr, Mn, Co, Al, which have proven useful for asymmetric transformations. For an example, see the Jacobsen epoxidation, which is catalyzed by a chiral manganese-salen complex:[4]
x
Complexes with salen-type ligands
[edit]The name “salen” or “salen-type” may be used for other ligands that have similar environment around the chelating site, namely two acidic hydroxyls and two Schiff base (aryl-imine) groups. These include the ligands abbreviated as salph, from the condensation of 1,2-phenylenediamine and salicylaldehyde. Other "Salen-type" metal complexes are formed with ligands with similar chelating groups, such as salph and salqu. Salqu copper complexes have been investigated as oxidation catalysts.[17]
salan or salalen ligands have one or two saturated nitrogen–aryl bonds (amines rather than imines). They are less rigid and more electron-rich at the metal center than the corresponding salen complexes.[18][19] Salans can be synthesized by the alkylation of an appropriate amine with a phenolic alkyl halide. The “half-salen” ligands have only one salicylimine group. They are prepared from a salicylaldehyde and a monoamine.[20]
Acacen ligands
[edit]
A class of tetradentate ligands with the generic name acacen are obtained by the condensation of derivatives of acetylacetone and ethylenediamine.[21]
Further reading
[edit]- Hazra, S.; Mohanta, S. (2019). "Metal–tin derivatives of compartmental Schiff Bases: Synthesis, structure and application". Coordination Chemistry Reviews. 395:1-24. https://doi.org/10.1016/j.ccr.2019.05.013
- McGarrigle, Eoghan M.; Gilheany, Declan G. (2005). "Chromium− and Manganese−salen Promoted Epoxidation of Alkenes". Chemical Reviews. 105 (5): 1563–1602. doi:10.1021/cr0306945. PMID 15884784.
- Bandini, Marco; Cozzi, Pier Giorgio; Umani-Ronchi, Achille (2002). "[Cr(Salen)] as a 'bridge' between asymmetric catalysis, Lewis acids and redox processes". Chemical Communications (9): 919–927. doi:10.1039/b109945k. PMID 12123051.
References
[edit]- ^ a b Tsumaki, T. (1938). "Nebenvalenzringverbindungen. IV. Über einige innerkomplexe Kobaltsalze der Oxyaldimine". Bulletin of the Chemical Society of Japan (in German). 13 (2): 252–260. doi:10.1246/bcsj.13.252.
- ^ Baleizão, Carlos; Garcia, Hermenegildo (2006). "Chiral Salen Complexes: An Overview to Recoverable and Reusable Homogeneous and Heterogeneous Catalysts". Chemical Reviews. 106 (9): 3987–4043. doi:10.1021/cr050973n. PMID 16967927.
- ^ Decortes, Antonello; Castilla, Ana M.; Kleij, Arjan W. (2010). "Salen-Complex-Mediated Formation of Cyclic Carbonates by Cycloaddition of CO2 to Epoxides". Angewandte Chemie International Edition. 49 (51): 9822–9837. doi:10.1002/anie.201002087. PMID 20957709.
- ^ a b c Larrow, J. F.; Jacobsen, E. N. (2004). "(R,R)-N,N'-Bis(3,5-Di-tert-Butylsalicylidene)-1,2-Cyclohexanediamino Manganese(III) Chloride, A Highly Enantioselective Epoxidation Catalyst". Organic Syntheses; Collected Volumes, vol. 10, p. 96.
- ^ Coggon, P.; McPhail, A. T.; Mabbs, F. E.; Richards, A.; Thornley, A. S. (1970). "Preparation, Magnetic, and Electronic Spectral Properties of Some Chromium(III)–NN′-Ethylenebis(salicylideneiminato) Complexes: Crystal and Molecular Structure of N,N′-Ethylenebis(salicylideneiminato)diaquochromium(III) Chloride". J. Chem. Soc. A: 3296–3303. doi:10.1039/j19700003296.
- ^ Nakajima, Kiyohiko; Kojima, Katsuhide; Kojima, Masaaki; Fujita, Junnosuke (1990). "Preparation and Characterization of Optically Active Schiff Base-Oxovanadium(IV) and -Oxovanadium(V) Complexes and Catalytic Properties of These Complexes on Asymmetric Oxidation of Sulfides into Sulfoxides with Organic Hydroperoxides". Bulletin of the Chemical Society of Japan. 63 (9): 2620–2630. doi:10.1246/bcsj.63.2620.
- ^ Lauffer, Randall B.; Heistand, Robert H.; Que, Lawrence (1983). "Dioxygenase models. Crystal Structures of the 2,4-Pentanedionato, Phenanthrenesemiquinone, and Catecholato Complexes of N,N′-Ethylenebis(salicylideneaminato)iron(III)". Inorganic Chemistry. 22: 50–55. doi:10.1021/ic00143a013.
- ^ Huilan, Chen; Deyan, Han; Tian, Li; Hong, Yan; Wenxia, Tang; Jian; Peiju; Chenggang (1996). "Synthesis and Crystal Structure of Organocobalt(III) Complexes with Secondary Alkyls or Bulky Schiff Base Equatorial Ligands". Inorganic Chemistry. 35 (6): 1502–1508. doi:10.1021/ic940516h. PMID 11666365.
- ^ Appleton, T. G. (1977). "Oxygen Uptake by a Cobalt(II) Complex". J. Chem. Educ. 54 (7): 443. doi:10.1021/ed054p443.
- ^ Yamada, Shoichiro (1999). "Advancement in stereochemical aspects of Schiff base metal complexes". Coordination Chemistry Reviews. 190–192: 537–555. doi:10.1016/S0010-8545(99)00099-5.
- ^ Dabelstein, W.; Reglitzky A.; Schutze A.; Reders, K. "Automotive Fuels". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_719.pub2. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - ^ a b Cozzi, Pier Giorgio (2004). "Metal–Salen Schiff base complexes in catalysis: Practical aspects". Chem. Soc. Rev. 33 (7): 410–21. doi:10.1039/B307853C. PMID 15354222.
- ^ Yoon, TP; Jacobsen, EN (2003). "Privileged Chiral Catalysts". Science. 299 (5613): 1691–1693. Bibcode:2003Sci...299.1691Y. doi:10.1126/science.1083622. PMID 12637734. S2CID 27416160.
- ^ Makoto Tokunaga; Jay F. Larrow; Fumitoshi Kakiuchi; Eric N. Jacobsen (1997). "Asymmetric Catalysis with Water: Efficient Kinetic Resolution of Terminal Epoxides by Means of Catalytic Hydrolysis". Science. 277 (5328): 936–938. doi:10.1126/science.277.5328.936. PMID 9252321. S2CID 23745844.
- ^ D. J. Darensbourg (2007). "Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2". Chemical Reviews. 107 (6): 2388–2410. doi:10.1021/cr068363q. PMID 17447821.
- ^ Dabelstein, W.; Reglitzky A.; Schutze A.; Reders, K. "Automotive Fuels". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_719.pub2. ISBN 978-3527306732.
- ^ Wu, Xianghong; Gorden, A. V. E. (2009). "2-Quinoxalinol Salen Copper Complexes for Oxidation of Aryl Methylenes". Eur. J. Org. Chem. 2009 (4): 503–509. doi:10.1002/ejoc.200800928.
- ^ Atwood, David A.; Remington, Michael P.; Rutherford, Drew (1996). "Use of the Salan Ligands to Form Bimetallic Aluminum Complexes". Organometallics. 15 (22): 4763. doi:10.1021/om960505r.
- ^ Berkessel, Albrecht; Brandenburg, Marc; Leitterstorf, Eva; Frey, Julia; Lex, Johann; Schäfer, Mathias (2007). "A Practical and Versatile Access to Dihydrosalen (Salalen) Ligands: Highly Enantioselective Titanium. In Situ Catalysts for Asymmetric Epoxidation with Aqueous Hydrogen Peroxide". Adv. Synth. Catal. 349 (14–15): 2385. doi:10.1002/adsc.200700221.
- ^ Pang, Xuan; Duan, Ranlong; Li, Xiang; Sun, Zhiqiang; Zhang, Han; Wang, Xianhong; Chen, Xuesi (2014). "Synthesis and characterization of half-salen complexes and their application in the polymerization of lactide and ε-caprolactone". Polymer Chemistry. 5 (23): 6857–6864. doi:10.1039/C4PY00734D.
- ^ a b Weber, Birgit; Jäger, Ernst-G. (2009). "Structure and Magnetic Properties of Iron(II/III) Complexes with N
2O2–
2-Coordinating Schiff Base-Like Ligands". Eur. J. Inorg. Chem.: 455. doi:10.1002/ejic.200990003.
