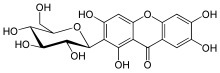
Mangiferin
| |

| |
| Names | |
|---|---|
| IUPAC name
2-(β-D-Glucopyranosyl)-1,3,6,7-tetrahydroxy-9H-xanthen-9-one
| |
| Systematic IUPAC name
1,3,6,7-Tetrahydroxy-2-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-9H-xanthen-9-one | |
| Other names
(1S)-1,5-Anhydro-1-(1,3,6,7-tetrahydroxy-9-oxo-9H-xanthen-2-yl)-D-glucitol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.153.319 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H18O11 | |
| Molar mass | 422.342 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Mangiferin is a glucosylxanthone (xanthonoid). This molecule is a glucoside of norathyriol.
Natural occurrences
[edit]Mangiferin was first isolated from the leaves and bark of Mangifera indica (the mango tree).[1] It can also be extracted from mango peels and kernels,[2][3] Iris unguicularis,[4] Anemarrhena asphodeloides rhizomes[5] and Bombax ceiba leaves.[6] It is also found in the genera Salacia and Cyclopia, as well as in coffee leaves and some species of Crocus.
Among the group of Asplenium hybrids known as the "Appalachian Asplenium complex", mangiferin and isomangiferin are produced only by Asplenium montanum and its hybrid descendants. The distinctive gold-orange fluorescence of these compounds under ultraviolet light has been used to aid in the chromatographic identification of hybrid Aspleniums.[7]
Research
[edit]Preliminary research is conducted on the potential biological properties of mangiferin,[8] although there were no confirmed anti-disease effects or prescription drugs approved, as of 2019.[9]
See also
[edit]References
[edit]- ^ K. Gorter (April 1922). "Sur La Substance Mère du Jaune Indien", Bulletin du Jardin botanique de Buitenzorg, (in French). Volume 4 Series 3 Issue 2: p. 260–267; [J.C.S. (20 April 1923). "The precursor of Indian-yellow", Chemical Abstracts, (in English), Volume 17 Issue No. 8: p. 1472] – via archive.com
- ^ Barreto J.C.; Trevisan M.T.S.; Hull W.E.; Erben G.; De Brito E.S.; Pfundstein B.; Würtele G.; Spiegelhalder B.; Owen R.W. (2008). "Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.)". Journal of Agricultural and Food Chemistry. 56 (14): 5599–5610. doi:10.1021/jf800738r. PMID 18558692.
- ^ Rajneet K Khurana, Rajneet K Khurana; Kaur, Ranjot; Lohan, Shikha; K Singh, Kamalinder; Singh, Bhupinder (2016). "Mangiferin: a promising anticancer bioactive" (PDF). Pharmaceutical Patent Analyst. 5 (3): 169–181. doi:10.4155/ppa-2016-0003. PMID 27088726.
- ^ Atta-Ur-Rahman; Hareem, Sumaira; Iqbal Choudhary, Muhammad; Sener, Bilge; Abbaskhan, Ahmed; Siddiqui, Hina; Anjum, Shazia; Orhan, Ilkay; Gurbuz, Ilhan; Ayanoglu, Filiz (2010). "New and Known Constituents from Iris unguicularis and Their Antoioxidant Activity". Heterocycles. 82: 813. doi:10.3987/COM-10-S(E)6.
- ^ Miura, T.; Ichiki, H.; Hashimoto, I.; Iwamoto, N.; Kato, M.; Kubo, M.; Ishihara, E.; Komatsu, Y.; Okada, M.; Ishida, T.; Tanigawa, K. (2001). "Antidiabetic Activity of a Xanthone Compound, Mangiferin". Phytomedicine. 8 (2): 85–87. doi:10.1078/0944-7113-00009. PMID 11315760.
- ^ Dar, A; Faizi, S; Naqvi, S; Roome, T; Zikr-Ur-Rehman, S; Ali, M; Firdous, S; Moin, S. T. (2005). "Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship". Biological & Pharmaceutical Bulletin. 28 (4): 596–600. doi:10.1248/bpb.28.596. PMID 15802793.
- ^ Smith, Dale M.; Harborne, Jeffrey B. (1971). "Xanthones in the Appalachian Asplenium complex". Phytochemistry. 10 (9): 2117–2119. Bibcode:1971PChem..10.2117S. doi:10.1016/S0031-9422(00)97205-4.
- ^ Khare, Puja; Shanker, Karuna (10 September 2016). "Mangiferin: A review of sources and interventions for biological activities". BioFactors. 42 (5): 504–514. doi:10.1002/biof.1308. ISSN 0951-6433. PMID 27658353. S2CID 31518535.
- ^ "Mangiferin, CID 5281647". PubChem, National Library of Medicine, US National Institutes of Health. 23 November 2019. Retrieved 24 November 2019.
