Drosera
| Drosera | |
|---|---|

| |
| Drosera tokaiensis | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Order: | Caryophyllales |
| Family: | Droseraceae |
| Genus: | Drosera L. |
| Subgenera | |
| Synonyms[1] | |
| |
Drosera, which is commonly known as the sundews, is one of the largest genera of carnivorous plants, with at least 194 species.[2] These members of the family Droseraceae[1] lure, capture, and digest insects using stalked mucilaginous glands covering their leaf surfaces. The insects are used to supplement the poor mineral nutrition of the soil in which the plants grow. Various species, which vary greatly in size and form, are native to every continent except Antarctica.[3]
Charles Darwin performed much of the early research into Drosera, engaging in a long series of experiments with Drosera rotundifolia which were the first to confirm carnivory in plants.[4][5][6] In an 1860 letter, Darwin wrote, “…at the present moment, I care more about Drosera than the origin of all the species in the world.”[7]
Taxonomy
[edit]The botanical name from the Greek δρόσος drosos "dew, dewdrops"[8] refer to the glistening drops of mucilage at the tip of the glandular trichomes that resemble drops of morning dew. The English common name sundew also describes this, derived from Latin ros solis meaning "dew of the sun".
The Principia Botanica, published in 1787, states “Sun-dew (Drosera) derives its name from small drops of a liquor-like dew, hanging on its fringed leaves, and continuing in the hottest part of the day, exposed to the sun.”[9]
Phylogenetic's
[edit]The unrooted cladogram to the right shows the relationship between various subgenera and classes as defined by the analysis of Rivadavia et al.[10] The monotypic section Meristocaulis was not included in the study, so its place in this system is unclear. More recent studies have placed this group near section Bryastrum, so it is placed there below. Also of note, the placement of the section Regiae in relation to Aldrovanda and Dionaea is uncertain. Since the section Drosera is polyphyletic, it shows up multiple times in the cladogram (*).
This phylogenetic study has made the need for a revision of the genus even clearer.
|
Description
[edit]
Sundews are perennial (or rarely annual) herbaceous plants, forming prostrate or upright rosettes between 1 and 100 cm (0.39 and 39.37 in) in height, depending on the species. Climbing species form scrambling stems which can reach much longer lengths, up to 3 m (9.8 ft) in the case of D. erythrogyne.[11] Sundews have been shown to be able to achieve a lifespan of 50 years.[12]: 102 The genus is specialized for nutrient uptake through its carnivorous behavior, for example the pygmy sundew is missing the enzymes (nitrate reductase, in particular)[13] that plants normally use for the uptake of earth-bound nitrates.
Growth Form
[edit]The genus can be divided into several habits, or growth forms:
- Temperate sundews: These species form a tight cluster of unfurled leaves called a hibernaculum in a winter dormancy period (= Hemicryptophyte). All of the North American and European species belong to this group. Drosera arcturi from Australia (including Tasmania) and New Zealand is another temperate species that dies back to a horn-shaped hibernaculum.
- Subtropical sundews: These species maintain vegetative growth year-round under uniform or nearly uniform climatic conditions.
- Pygmy sundews: A group of roughly 40 Australian species, they are distinguished by miniature growth, the formation of gemmae for asexual reproduction, and dense formation of hairs in the crown center. These hairs serve to protect the plants from Australia's intense summer sun. Pygmy sundews form the subgenus Bryastrum.
- Tuberous sundews: These nearly 50 Australian species form an underground tuber to survive the extremely dry summers of their habitat, re-emerging in the autumn. These so-called tuberous sundews can be further divided into two groups, those that form rosettes and those that form climbing or scrambling stems. Tuberous sundews comprise the subgenus Ergaleium.

- Petiolaris complex: A group of tropical Australian species, they live in constantly warm but sometimes wet conditions. Several of the 14 species that comprise this group have developed special strategies to cope with the alternately drier conditions. Many species, for example, have petioles densely covered in trichomes, which maintain a sufficiently humid environment and serve as an increased condensation surface for morning dew. The Petiolaris complex comprises the subgenus Lasiocephala.
Although they do not form a single strictly defined growth form, a number of species are often put together in a further group:
- Queensland sundews: A small group of three species (D. adelae, D. schizandra and D. prolifera), all are native to highly humid habitats in the dim understories of the Australian rainforest.
Leaves and Entomophagy
[edit]
Sundews are characterised by the glandular tentacles, topped with sticky secretions, that cover their leaves. The trapping and digestion mechanism usually employs two types of glands: stalked glands that secrete sweet mucilage to attract and ensnare insects and enzymes to digest them, and sessile glands that absorb the resulting nutrient soup (the latter glands are missing in some species, such as D. erythrorhiza). Small prey, mainly consisting of insects, are attracted by the sweet secretions of the peduncular glands. Upon touching these, the prey become entrapped by sticky mucilage which prevents their progress or escape. Eventually, the prey either succumb to death through exhaustion or through asphyxiation as the mucilage envelops them and clogs their spiracles. Death usually occurs within 15 minutes.[6] The plant meanwhile secretes esterase, peroxidase, phosphatase and protease enzymes.[12]: 41 These enzymes dissolve the insect and free the nutrients contained within it. This nutrient mixture is then absorbed through the leaf surfaces to be used by the rest of the plant.

All species of sundew are able to move their tentacles in response to contact with edible prey. The tentacles are extremely sensitive and will bend toward the center of the leaf to bring the insect into contact with as many stalked glands as possible. According to Charles Darwin, the contact of the legs of a small gnat with a single tentacle is enough to induce this response.[6] This response to touch is known as thigmonasty, and is quite rapid in some species. The outer tentacles (recently coined as "snap-tentacles") of D. burmanni and D. sessilifolia can bend inwards toward prey in a matter of seconds after contact, while D. glanduligera is known to bend these tentacles in toward prey in tenths of a second.[14] In addition to tentacle movement, some species are able to bend their leaves to various degrees to maximize contact with the prey. Of these, D. capensis exhibits what is probably the most dramatic movement, curling its leaf completely around prey in 30 minutes. Some species, such as D. filiformis, are unable to bend their leaves in response to prey.[15]

A further type of (mostly strong red and yellow) leaf coloration has recently been discovered in a few Australian species (D. hartmeyerorum, D. indica). Their function is not known yet, although they may help in attracting prey.
The leaf morphology of the species within the genus is extremely varied, ranging from the sessile ovate leaves of D. erythrorhiza to the bipinnately divided acicular leaves of D. binata.
While the exact physiological mechanism of the sundew's carnivorous response is not yet known, some studies have begun to shed light on how the plant is able to move in response to mechanical and chemical stimulation to envelop and digest prey. Individual tentacles, when mechanically stimulated, fire action potentials that terminate near the base of the tentacle, resulting in rapid movement of the tentacle towards the center of the leaf.[16][17] This response is more prominent when marginal tentacles further away from the leaf center are stimulated. The tentacle movement response is achieved through auxin-mediated acid growth. When action potentials reach their target cells, the plant hormone auxin causes protons (H+ ions) to be pumped out of the plasma membrane into the cell wall, thereby reducing the pH and making the cell wall more acidic.[18] The resulting reduction in pH causes the relaxation of the cell wall protein, expansin, and allows for an increase in cell volume via osmosis and turgor. As a result of differential cell growth rates, the sundew tentacles are able to achieve movement towards prey and the leaf center through the bending caused by expanding cells.[19] Among some drosera species, a second bending response occurs in which non-local, distant tentacles bend towards prey as well as the bending of the entire leaf blade to maximize contact with prey. While mechanical stimulation is sufficient to achieve a localized tentacle bend response, both mechanical and chemical stimuli are required for the secondary bending response to occur.[20]
Flowers and fruit
[edit]
The flowers of sundews, as with nearly all carnivorous plants, are held far above the leaves by a long stem. This physical isolation of the flower from the traps is commonly thought to be an adaptation meant to avoid trapping potential pollinators. The mostly unforked inflorescences are spikes, whose flowers open one at a time and usually only remain open for a short period. Flowers open in response to light intensity (often opening only in direct sunlight), and the entire inflorescence is also heliotropic, moving in response to the sun's position in the sky.

The radially symmetrical (actinomorphic) flowers are always perfect and have five parts (the exceptions to this rule are the four-petaled D. pygmaea and the eight to 12-petaled D. heterophylla). Most of the species have small flowers (<1.5 cm or 0.6 in). A few species, however, such as D. regia and D. cistiflora, have flowers 4 cm (1.6 in) or more in diameter.[15] In general, the flowers are white or pink. Australian species display a wider range of colors, including orange (D. callistos), red (D. adelae), yellow (D. zigzagia) or metallic violet (D. microphylla).
The ovary is superior and develops into a dehiscent seed capsule bearing numerous tiny seeds. The pollen grain type is compound, which means four microspores (pollen grains) are stuck together with a protein called callose.[citation needed]
Roots
[edit]
The root systems of most Drosera are often only weakly developed or have lost their original functions.[21] They are relatively useless for nutrient uptake, and they serve mainly to absorb water and to anchor the plant to the ground; they have long hairs.[21]
A few South African species use their roots for water and food storage. Some species have wiry root systems that remain during frosts if the stem dies. Some species, such as D. adelae and D. hamiltonii, use their roots for asexual propagation, by sprouting plantlets along their length. Some Australian species form underground corms for this purpose, which also serve to allow the plants to survive dry summers. The roots of pygmy sundews are often extremely long in proportion to their size, with a 1-cm (0.4-in) plant extending roots over 15 cm (5.9 in) beneath the soil surface. Some pygmy sundews, such as D. lasiantha and D. scorpioides, also form adventitious roots as supports.
D. intermedia and D. rotundifolia have been reported to form arbuscular mycorrhizae, which penetrate the plant's tissues,[22] they also host fungi like endophytes to collect nutrients when they grow in poor soil and form symbiotic relationships.[23]
Reproduction
[edit]Many species of sundews are self-fertile; their flowers will often self-pollinate upon closing. Often, numerous seeds are produced. The tiny black seeds germinate in response to moisture and light, while seeds of temperate species also require cold, damp, stratification to germinate. Seeds of the tuberous species require a hot, dry summer period followed by a cool, moist winter to germinate.
Vegetative reproduction occurs naturally in some species that produce stolons or when roots come close to the surface of the soil. Older leaves that touch the ground may sprout plantlets. Pygmy sundews reproduce asexually using specialized scale-like leaves called gemmae. Tuberous sundews can produce offsets from their corms.[15]
In culture, sundews can often be propagated through leaf, crown, or root cuttings, as well as through seeds.[15]
Distribution
[edit]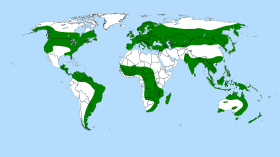

The range of the sundew genus stretches from Alaska in the north to New Zealand in the south. The centers of diversity are Australia, with roughly 50% of all known species, and South America and southern Africa, each with more than 20 species. A few species are also found in large parts of Eurasia and North America. These areas, however, can be considered to form the outskirts of the generic range, as the ranges of sundews do not typically approach temperate or Arctic areas. Contrary to previous supposition, the evolutionary speciation of this genus is no longer thought to have occurred with the breakup of Gondwana through continental drift. Rather, speciation is now thought to have occurred as a result of a subsequent wide dispersal of its range.[10] The origins of the genus are thought to have been in Africa or Australia.[10]
Europe is home to only three species: D. intermedia, D. anglica, and D. rotundifolia. Where the ranges of the two latter species overlap, they sometimes hybridize to form the sterile D. × obovata. In addition to the three species and the hybrid native to Europe, North America is also home to four additional species; D. brevifolia is a small annual native to coastal states from Texas to Virginia, while D. capillaris, a slightly larger plant with a similar range, is also found in areas of the Caribbean. The third species, D. linearis, is native to the northern United States and southern Canada. D. filiformis has two subspecies native to the East Coast of North America, the Gulf Coast, and the Florida panhandle.
This genus is often described as cosmopolitan, meaning it has worldwide distribution. The botanist Ludwig Diels, author of the only monograph of the family to date, called this description an "arrant misjudgment of this genus' highly unusual distributional circumstances (arge Verkennung ihrer höchst eigentümlichen Verbreitungsverhältnisse)", while admitting sundew species do "occupy a significant part of the Earth's surface (einen beträchtlichen Teil der Erdoberfläche besetzt)".[24] He particularly pointed to the absence of Drosera species from almost all arid climate zones, countless rainforests, the American Pacific Coast, Polynesia, the Mediterranean region, and North Africa, as well as the scarcity of species diversity in temperate zones, such as Europe and North America.[24]
Habitat
[edit]
Sundews generally grow in seasonally moist or more rarely constantly wet habitats with acidic soils and high levels of sunlight. Common habitats include bogs, fens, swamps, marshes, the tepuis of Venezuela, the wallums of coastal Australia, the fynbos of South Africa, and moist streambanks. Many species grow in association with sphagnum moss, which absorbs much of the soil's nutrient supply and also acidifies the soil, making nutrients less available to plant life. This allows sundews, which do not rely on soil-bound nutrients, to flourish where more dominating vegetation would usually outcompete them.
The genus, though, is very variable in terms of habitat. Individual sundew species have adapted to a wide variety of environments, including atypical habitats, such as rainforests, deserts (D. burmanni and D. indica), and even highly shaded environments (Queensland sundews). The temperate species, which form hibernacula in the winter, are examples of such adaptation to habitats; in general, sundews tend to inhabit warm climates, and are only moderately frost-resistant.
Conservation status
[edit]
Protection of the genus varies between countries. None of the Drosera species in the United States are federally protected. Some are listed as threatened or endangered at the state level, but this gives little protection to lands under private ownership.[25] Many of the remaining native populations are located on protected land, such as national parks or wildlife preserves.[26] Drosera species are protected by law in many European countries, such as Germany, Austria, Switzerland, the Czech Republic, Finland, Hungary, France, and Bulgaria.[27] In Australia, they are listed as "threatened".[28] In South America and the Caribbean, Drosera species in a number of areas are considered critical, endangered or vulnerable, while other areas have not been surveyed.[29] At the same time that species are at risk in South Africa,[30] new species continue to be discovered in the Western Cape[31] and Madagascar.[32]
Worldwide, Drosera are at risk of extinction due to the destruction of natural habitat through urban and agricultural development. They are also threatened by the illegal collection of wild plants for the horticultural trade.[30] An additional risk is environmental change, because species are often specifically adapted to a precise location and set of conditions.[30]
Currently, the largest threat in Europe and North America is loss of wetland habitat.[25][33] Causes include urban development and the draining of bogs for agricultural uses and peat harvesting.[30] Such threats have led to the extirpation of some species from parts of their former range. Reintroduction of plants into such habitats is usually difficult or impossible, as the ecological needs of certain populations are closely tied to their geographical location.[30] Increased legal protection of bogs and moors,[34] and a concentrated effort to renaturalize such habitats, are possible ways to combat threats to Drosera plants' survival.[35] As part of the landscape, sundews are often overlooked or not recognized at all.[36][37]
In South Africa and Australia, two of the three centers of species diversity, the natural habitats of these plants are undergoing a high degree of pressure from human activities. The African sundews D. insolita and D. katangensis are listed as critically endangered by the International Union for Conservation of Nature (IUCN), while D. bequaertii is listed as vulnerable.[25] Expanding population centers such as Queensland, Perth, and Cape Town, and the draining of moist areas for agriculture and forestry in rural areas threaten many such habitats. The droughts that have been sweeping Australia in the 21st century pose a threat to many species by drying up previously moist areas.[30]
Those species endemic to a very limited area are often most threatened by the collection of plants from the wild. D. madagascariensis is considered endangered in Madagascar because of the large-scale removal of plants from the wild for exportation; 10 - 200 million plants are harvested for commercial medicinal use annually.[27][30]
Gallery of prey
[edit]-
Tipulidae (crane fly) trapped by Drosera filiformis
-
Moth, Phalaenophana pyramusalis (Dark-banded Owlet) trapped by Drosera filiformis
-
Eusarca confusaria moth trapped by Drosera filiformis
-
Tabanus fly trapped by Drosera filiformis
-
Drosophila melanogaster fly trapped by Drosera capensis
Uses
[edit]Traditional medicine
[edit]Sundews were used as medicinal herbs as early as the 12th century, when an Italian doctor from the School of Salerno, Matthaeus Platearius, described the plant as an herbal remedy for coughs under the name herba sole.[38] Culbreth's 1927 Materia Medica listed D. rotundifolia, D. anglica and D. linearis as being used as stimulants and expectorants, and "of doubtful efficacy" for treating bronchitis, whooping cough, and tuberculosis.[39] Sundew tea was recommended by herbalists for dry coughs, bronchitis, whooping cough, asthma and "bronchial cramps".[40] The French Pharmacopoeia of 1965 listed sundew for the treatment of inflammatory diseases such as asthma, chronic bronchitis and whooping cough.[41]
Drosera has been used commonly in cough preparations in Germany and elsewhere in Europe.[42] In traditional medicine practices, Drosera is used to treat ailments such as asthma, coughs, lung infections, and stomach ulcers.[43]
Herbal preparations are primarily made using the roots, flowers, and fruit-like capsules.[44] Since all native sundews species are protected in many parts of Europe and North America, extracts are usually prepared using cultivated fast-growing sundews (specifically D. rotundifolia, D. intermedia, D. anglica, D. ramentacea and D. madagascariensis) or from plants collected and imported from Madagascar, Spain, France, Finland and the Baltics.
Sundews are historically mentioned as an aphrodisiac[45] (hence the common name lustwort).[46] They are mentioned as a folk remedy for treatment of warts, corns, and freckles.[47]
As ornamental plants
[edit]Because of their carnivorous nature and the beauty of their glistening traps, sundews have become favorite ornamental plants; however, the environmental requirements of most species are relatively stringent and can be difficult to meet in cultivation. As a result, most species are unavailable commercially. A few of the hardiest varieties, however, have made their way into the mainstream nursery business and can often be found for sale next to Venus flytraps. These most often include D. capensis, D. aliciae, and D. spatulata.[48]
Cultivation requirements vary greatly by species. In general, though, sundews require high environmental moisture content, usually in the form of a constantly moist or wet soil substrate. Most species also require this water to be pure, as nutrients, salts, or minerals in their soil can stunt their growth or even kill them. Commonly, plants are grown in a soil substrate containing some combination of dead or live sphagnum moss, sphagnum peat moss, sand, and/or perlite, and are watered with distilled, reverse osmosis, or rain water.[15]
Nano-biotechnology
[edit]The mucilage produced by Drosera has remarkable elastic properties and has made this genus a very attractive subject in biomaterials research. In one recent study, the adhesive mucilages of three species (D. binata, D. capensis, and D. spatulata) were analyzed for nanofiber and nanoparticle content.[49] Using atomic force microscopy, transmission electron microscopy, and energy-dispersive X-ray spectroscopy, researchers were able to observe networks of nanofibers and nanoparticles of various sizes within the mucilage residues. In addition, calcium, magnesium, and chlorine – key components of biological salts - were identified.[49] These nanoparticles are theorized to increase the viscosity and stickiness of the mucilage, in turn increasing the effectiveness of the trap.
More importantly for biomaterials research, however, is the fact that, when dried, the mucin provides a suitable substrate for the attachment of living cells. This has important implications for tissue engineering, especially because of the elastic qualities of the adhesive. Essentially, a coating of Drosera mucilage on a surgical implant, such as a replacement hip or an organ transplant, could drastically improve the rate of recovery and decrease the potential for rejection, because living tissue can effectively attach and grow on it. The authors also suggest a wide variety of applications for Drosera mucin, including wound treatment, regenerative medicine, or enhancing synthetic adhesives.[49] Because this mucilage can stretch to nearly a million times its original size and is readily available for use, it can be an extremely cost-efficient source of biomaterial.[50]
Other uses
[edit]The corms of the tuberous sundews native to Australia are considered a delicacy by the Indigenous Australians.[12]: 100 Some of these corms were also used to dye textiles,[51][52] while another purple or yellow dye was traditionally prepared in the Scottish Highlands using D. rotundifolia.[53] A sundew liqueur is also still produced using a recipe from the 14th century. It is made using fresh leaves from mainly D. capensis, D. spatulata, and D. rotundifolia.[52]
Chemical constituents
[edit]Several chemical compounds with potential biological activities are found in sundews, including flavonoids (kaempferol, myricetin, quercetin and hyperoside),[54] quinones (plumbagin,[55] hydroplumbagin glucoside[56] and rossoliside (7–methyl–hydrojuglone–4–glucoside)[57]), and other constituents such as carotenoids, plant acids (e.g. butyric acid, citric acid, formic acid, gallic acid, malic acid, propionic acid), resin, tannins and ascorbic acid (vitamin C).
References
[edit]- ^ a b "Drosera L." Plants of the World Online. Royal Botanic Gardens, Kew. 2023. Retrieved 13 January 2023.
- ^ McPherson, S.R. (2010). Carnivorous Plants and their Habitats. Poole, England, UK: Redfern Natural History Productions Ltd. 2 volumes.
- ^ McPherson, S.R. (2008). Glistening Carnivores. Poole, England, UK: Redfern Natural History Productions Ltd.
- ^ Pain, Stephanie (2 March 2022). "How plants turned predator". Knowable Magazine. doi:10.1146/knowable-030122-1. Retrieved 11 March 2022.
- ^ Hedrich, Rainer; Fukushima, Kenji (17 June 2021). "On the Origin of Carnivory: Molecular Physiology and Evolution of Plants on an Animal Diet". Annual Review of Plant Biology. 72 (1): 133–153. doi:10.1146/annurev-arplant-080620-010429. ISSN 1543-5008. PMID 33434053. S2CID 231595236.
- ^ a b c Darwin, Charles (1875). Insectivorous Plants. London: John Murray. Retrieved March 14, 2022.
- ^ Ellison, Aaron M.; Gotelli, Nicholas J. (1 January 2009). "Energetics and the evolution of carnivorous plants—Darwin's 'most wonderful plants in the world'". Journal of Experimental Botany. 60 (1): 19–42. doi:10.1093/jxb/ern179. ISSN 0022-0957. PMID 19213724.
- ^ Liddell, Henry George; Scott, Robert (1940). "δρόσος". A Greek-English Lexicon. Perseus Digital Library.
- ^ Darwin, the Elder, Robert Waring (1787). Principia Botanica; Or, A Concise and Easy Introduction to the Sexual Botany of Linnæus, Etc. [The Preface Signed: R. W. D., I.e. Robert W. Darwin, the Elder.]. Newark: Allin & Company. Retrieved 14 March 2022.
- ^ a b c Rivadavia, Fernando; Kondo, Katsuhiko; Kato, Masahiro & Hasebe, Mitsuyasu (2003). "Phylogeny of the sundews, Drosera (Droseraceae), based on chloroplast rbcL and nuclear 18S ribosomal DNA Sequences". American Journal of Botany. 90 (1): 123–130. doi:10.3732/ajb.90.1.123. PMID 21659087.
- ^ Mann, Phill (2001). "The world's largest Drosera". Carnivorous Plant Newsletter. 30 (3): 79. Archived from the original on 2011-09-29. Retrieved 2022-08-17.
- ^ a b c Barthlott, Wilhelm; Porembski, Stefan; Seine, Rüdiger; Theisen, Inge (1 June 2004). Karnivoren Biologie und Kultur fleischfressender Pflanzen. Stuttgart (Hohenheim): Verlag Eugen Ulmer. ISBN 9783800141449.
- ^ Karlsson, PS; Pate, JS (1992). "Contrasting effects of supplementary feeding of insects or mineral nutrients on the growth and nitrogen and phosphorous economy of pygmy species of Drosera". Oecologia. 92 (1): 8–13. Bibcode:1992Oecol..92....8K. doi:10.1007/BF00317256. PMID 28311806. S2CID 13038192.
- ^ Hartmeyer, I. & Hartmeyer, S., (2005) Drosera glanduligera: Der Sonnentau mit "Schnapp-Tentakeln", DAS TAUBLATT (GFP) 2005/2: 34-38
- ^ a b c d e D'Amato, Peter (1998). The Savage Garden: Cultivating Carnivorous Plants. Berkeley, California: Ten Speed Press. ISBN 978-0-89815-915-8.
- ^ Williams, Stephen E.; Pickard, Barbara G. (1972). "Receptor potentials and action potentials in Drosera tentacles". Planta. 103 (3): 193–221. Bibcode:1972Plant.103..193W. doi:10.1007/bf00386844. ISSN 0032-0935. PMID 24481555. S2CID 2155695.
- ^ Williams, S. E.; Pickard, B. G. (1980). "The Role of Action Potentials in the Control of Capture Movements of Drosera and Dionaea". Plant Growth Substances 1979. Proceedings in Life Sciences. pp. 470–480. doi:10.1007/978-3-642-67720-5_48. ISBN 978-3-642-67722-9.
- ^ Rayle, D. L.; Cleland, R. E. (1992-08-01). "The Acid Growth Theory of auxin-induced cell elongation is alive and well". Plant Physiology. 99 (4): 1271–1274. doi:10.1104/pp.99.4.1271. ISSN 0032-0889. PMC 1080619. PMID 11537886.
- ^ Hooker, Henry D. (1917). "Mechanics of Movement in Drosera rotundifolia". Bulletin of the Torrey Botanical Club. 44 (8): 389–403. doi:10.2307/2479748. JSTOR 2479748.
- ^ Krausko, Miroslav; Perutka, Zdeněk; Šebela, Marek; Šamajová, Olga; Šamaj, Jozef; Novák, Ondřej; Pavlovič, Andrej (March 2017). "The role of electrical and jasmonate signalling in the recognition of captured prey in the carnivorous sundew plant Drosera capensis". The New Phytologist. 213 (4): 1818–1835. doi:10.1111/nph.14352. ISSN 1469-8137. PMID 27933609.
- ^ a b Adlassnig, Wolfram; Peroutka, Marianne; Lambers, Hans; Lichtscheidl, Irene K. (July 2005). "The Roots of Carnivorous Plants". Plant and Soil. 274 (1–2): 127–140. Bibcode:2005PlSoi.274..127A. doi:10.1007/s11104-004-2754-2. S2CID 5038696.
- ^ Wang, B.; Qiu, Y.-L. (July 2006). "Phylogenetic distribution and evolution of mycorrhizas in land plants". Mycorrhiza. 16 (5): 299–363. Bibcode:2006Mycor..16..299W. doi:10.1007/s00572-005-0033-6. PMID 16845554. S2CID 30468942.
- ^ Quilliam, Richard S.; Jones, David L. (June 2010). "Fungal root endophytes of the carnivorous plant Drosera rotundifolia". Mycorrhiza. 20 (5): 341–348. Bibcode:2010Mycor..20..341Q. doi:10.1007/s00572-009-0288-4. PMID 20012108. S2CID 11825262.
- ^ a b Diels, Friedrich Ludwig Emil (1906). Engler, Adolf (ed.). "Droseraceae". Das Pflanzenreich. IV (112). Retrieved 14 March 2022.
- ^ a b c "Sundew". San Diego Zoo Animals & Plants. Retrieved 15 March 2022.
- ^ "Drosera anglica". NatureServe Explorer 2.0. Retrieved 15 March 2022.
- ^ a b Baranyai, B.; Jooste, H. (2016). "Biology, ecology, use, conservation and cultivation of round-leaved sundew (Drosera rotundifolia L.): a review" (PDF). Mires and Peat. 18: 1–28.
- ^ "Drosera prolifera Glossary SPRAT Profile". Species Profile and Threats Database. Australian Government. Retrieved 15 March 2022.
- ^ de Stefano, Rodrigo Duno; dos Santos Silva, Tania Regina (2001). "Conservation status of the carnivorous genus Drosera in South America and the Caribbean". Harvard Papers in Botany. 6 (1): 253–260. ISSN 1043-4534. JSTOR 41761649. Retrieved 15 March 2022.
- ^ a b c d e f g Cross, Adam T.; Krueger, Thilo A.; Gonella, Paulo M.; Robinson, Alastair S.; Fleischmann, Andreas S. (1 December 2020). "Conservation of carnivorous plants in the age of extinction". Global Ecology and Conservation. 24: e01272. Bibcode:2020GEcoC..2401272C. doi:10.1016/j.gecco.2020.e01272. hdl:20.500.11937/84611. ISSN 2351-9894. S2CID 225228358.
The most significant threats placing species at imminent risk of extinction include the continuing clearing of natural habitat for urban and agricultural development and the illegal collection of individuals from the wild for horticultural trade.
- ^ Fleischmann, Andreas (April 2018). "Drosera xerophila ( Droseraceae ), a new species from Overberg District, South Africa, and an overview of the rosetted hemicryptophyte sundew species from Western Cape Province". Willdenowia. 48 (1): 93–107. doi:10.3372/wi.48.48106. S2CID 90715895.
- ^ Fleischmann, Andreas S.; Rakotoarivelo, Nivo H.; Roccia, Aymeric; Gonella, Paulo M.; Andriamiarisoa, Lala Roger; Razanatsima, Aina; Rakotoarivony, Fortunat (2020). "A new and endemic species of Drosera (Droseraceae) from Madagascar". Plant Ecology and Evolution. 153 (2): 283–291. doi:10.5091/plecevo.2020.1705. JSTOR 26927031. S2CID 225539874.
- ^ "Sundews". The National Wildlife Federation. Retrieved 15 March 2022.
- ^ Lausche, Barbara J. (2011). Guidelines for Protected Areas Legislation. Gland, Switzerland: IUCN. p. 143. ISBN 978-2-8317-1245-1.
- ^ Barbour, Michael; Keeler-Wolf, Todd; Schoenherr, Allan A. (17 July 2007). Terrestrial Vegetation of California, 3rd Edition. University of California Press. ISBN 978-0-520-24955-4.
- ^ Newlin, William V. P.; Cline, Kenneth S.; Briggs, Rachel; Namnoum, A. Addison; Ciccotelli, Brett (27 August 2013). The College of the Atlantic Guide to the Lakes and Ponds of Mt. Desert: Discovering the Freshwater Gems of Maine's Largest Island. North Atlantic Books. ISBN 978-1-58394-797-5.
- ^ Harris, Kerri-Lee (August 25, 2020). "Rainbow Sundews". Life in a Southern Forest. Retrieved 15 March 2022.
- ^ "Drosera herba". Altmeyers Encyclopedia - Department Phytotherapy. 29 October 2020. Retrieved 17 March 2022.
- ^ Culbreth, David M. R. (1927). Materia Medica and Pharmacology. Philadelphia: Lea & Febiger.
- ^ Schilcher, H.; Elzer, M. (1993). "Drosera (Sundew): A proven antitussive". Zeitschrift für Phytotherapie. 14 (50): 4.
- ^ Ghate, N. B.; Das, A.; Chaudhuri, D.; Panja, S.; Mandal, N. (2016). "Sundew plant, a potential source of anti-inflammatory agents, selectively induces G2/M arrest and apoptosis in MCF-7 cells through upregulation of p53 and Bax/Bcl-2 ratio". Cell Death Discovery. 2: 15062. doi:10.1038/cddiscovery.2015.62. ISSN 2058-7716. PMC 4979533. PMID 27551490.
- ^ "Sundew". Kaiser Permanente. Retrieved 17 March 2022.
- ^ Hoffmann, David (24 October 2003). Medical Herbalism: The Science and Practice of Herbal Medicine. Simon and Schuster. ISBN 978-1-59477-890-2. Retrieved 17 March 2022.
- ^ Wichtl, M. (1994). Herbal drugs and phytopharmaceuticals : a handbook for practice on a scientific basis. Boca Raton, FL: CRC Press. pp. 178, 81.
- ^ Wolf, E.; Gage, E.; Cooper, D.J. (29 June 2006). "Drosera rotundifolia L. (roundleaf sundew): a technical conservation assessment" (PDF). USDA Forest Service, Rocky Mountain Region. Retrieved 17 March 2022.
- ^ Vogel, A. "Drosera rotundifolia L. | Round-leafed Sundew". Plant Encyclopaedia. Retrieved 17 March 2022.
- ^ Crowder, A. A.; Pearson, M. C.; Grubb, P. J.; Langlois, P. H. (1990). "Drosera L." Journal of Ecology. 78 (1): 233–267. Bibcode:1990JEcol..78..233C. doi:10.2307/2261048. ISSN 0022-0477. JSTOR 2261048. Retrieved 15 March 2022.
- ^ Rice, Barry. 2006. Growing Carnivorous Plants. Timber Press: Portland, Oregon.
- ^ a b c Zhang, M.; Lenaghan, S.C.; Xia, L.; Dong, L.; He, W.; Henson, W.R.; Fan, X. (2010). "Nanofibers and nanoparticles from the insect capturing adhesive of the Sundew (Drosera) for cell attachment". Journal of Nanobiotechnology. 8 (20): 20. doi:10.1186/1477-3155-8-20. PMC 2931452. PMID 20718990.
- ^ Gaddam, Susmila Aparna; Kotakadi, Venkata Subbaiah; Subramanyam, Gunasekhar Kalavakunta; Penchalaneni, Josthna; Challagundla, Varadarajulu Naidu; Dvr, Sai Gopal; Pasupuleti, Visweswara Rao (9 November 2021). "Multifaceted phytogenic silver nanoparticles by an insectivorous plant Drosera spatulata Labill var. bakoensis and its potential therapeutic applications". Scientific Reports. 11 (1): 21969. Bibcode:2021NatSR..1121969G. doi:10.1038/s41598-021-01281-8. ISSN 2045-2322. PMC 8578548. PMID 34753977. Retrieved 17 March 2022.
- ^ Yusuf, Mohd; Shabbir, Mohd; Mohammad, Faqeer (16 January 2017). "Natural Colorants: Historical, Processing and Sustainable Prospects". Natural Products and Bioprospecting. 7 (1): 123–145. doi:10.1007/s13659-017-0119-9. ISSN 2192-2195. PMC 5315675. PMID 28093670.
- ^ a b Plantarara (2001): Artzneimittle, Tee, und Likör aus fleischfressenden Pflanzen Archived 2006-06-18 at the Wayback Machine
- ^ Dwelly, Edward; "Dwelly’s [Scottish] Gaelic Dictionary" (1911) (Dath)
- ^ Ayuga C; et al. (1985). "Contribución al estudio de flavonoides en D. rotundifolia L". An R Acad Farm. 51: 321–326.
- ^ Wagner H; et al. (1986). "Immunological investigations of naphthoquinone – containing plant extracts, isolated quinones and other cytostatic compounds in cellular immunosystems". Phytochem Soc Eur Symp: 43.
- ^ Vinkenborg, J; Sampara-Rumantir, N; Uffelie, OF (1969). "The presence of hydroplumbagin glucoside in Drosera rotundifolia L". Pharmaceutisch Weekblad. 104 (3): 45–9. PMID 5774641.
- ^ Sampara-Rumantir N. (1971). "Rossoliside". Pharm Weekbl. 106 (35): 653–664. PMID 5566922.
Further reading
[edit]- Barthlott, Wilhelm; Porembski, Stefan; Seine, Rüdiger; Theisen, Inge (2007). The curious world of carnivorous plants : a comprehensive guide to their biology and cultivation (English language ed.). Portland, Or.: Timber Press. ISBN 978-0881927924.
- Correa A., Mireya D.; Silva, Tania Regina Dos Santos: Drosera (Droseraceae), in: Flora Neotropica, Monograph 96, New York, 2005
- Lowrie, Allen: Carnivorous Plants of Australia, Vol. 1–3, English, Nedlands, Western Australia, 1987–1998
- Lowrie, A. (2005). "A taxonomic revision of Drosera section Stolonifera (Droseraceae), from south-west Western Australia" (PDF). Nuytsia. 15 (3): 355–393. doi:10.58828/nuy00412.
- Olberg, Günter: Sonnentau, Natur und Volk, Bd. 78, Heft 1/3, pp. 32–37, Frankfurt, 1948
- Seine, Rüdiger; Barthlott, Wilhelm: Some proposals on the infrageneric classification of Drosera L., Taxon 43, 583 - 589, 1994
- Schlauer, Jan: A dichotomous key to the genus Drosera L. (Droseraceae), Carnivorous Plant Newsletter, Vol. 25 (1996)
External links
[edit]- A key to Drosera species, with distribution maps and growing difficulty scale
- A virtually exhaustive listing of Drosera pictures on the web
- International Carnivorous Plant Society
- Carnivorous Plant FAQ
- The Sundew Grow Guides
- Sundew images from smugmug
- Botanical Society of America, Drosera - the Sundews Archived 2010-06-13 at the Wayback Machine





