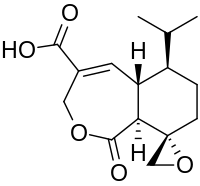
Koningic acid
Appearance
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2′S,5aS,6R,9aS)-1-Oxo-6-(propan-2-yl)-1,5a,6,7,8,9a-hexahydro-3H-spiro[[2]benzoxepine-9,2′-oxirane]-4-carboxylic acid | |
| Other names
(+)-Heptelidic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H20O5 | |
| Molar mass | 280.320 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Koningic acid (KA, also known as heptelidic acid) is a potent, selective, irreversible GAPDH inhibitor.[1][2] It is also a DNA polymerase inhibitor. The koningic acid molecule, produced by fungi that consume sweet potatoes, has been shown to curb the excessive glucose consumption in tumors exhibiting the Warburg effect and leaving healthy cells alone.[3]
References
[edit]- ^ "Heptelidic acid (Koningic acid) - Abcam". www.abcam.com.
- ^ Endo, Akira; Hasum, Kenji; Hasumi, Kenji; Sakai, Kaoru; Kanbe, Tomomi (1985). "Specific inhibition of glyceraldehyde-3-phosphate dehydrogenase by koningic acid (Heptelidic acid)". The Journal of Antibiotics. 38 (7): 920–5. doi:10.7164/antibiotics.38.920. PMID 4030504.
- ^ "Natural molecule appears to shut off cancer cells' energy source".
