Folinic acid
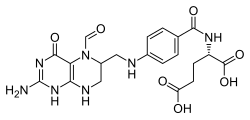 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | Leucovorin /ˌljuːkoʊˈvɔːrɪn/ |
| Trade names | Many |
| Other names | citrovorum factor, 5-formyltetrahydrofolate |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608038 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous, IM, by mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Dose dependent |
| Protein binding | ~15% |
| Elimination half-life | 6.2 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.328 |
| Chemical and physical data | |
| Formula | C20H23N7O7 |
| Molar mass | 473.446 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 245 °C (473 °F) decomp |
| Solubility in water | ~0.3[1] mg/mL (20 °C) |
| |
| |
| | |
Folinic acid, also known as leucovorin, is a medication used to decrease the toxic effects of methotrexate and pyrimethamine.[2][3] It is also used in combination with 5-fluorouracil to treat colorectal cancer and pancreatic cancer, may be used to treat folate deficiency that results in anemia, and methanol poisoning.[3][4] It is taken by mouth, injection into a muscle, or injection into a vein.[3]
Side effects may include trouble sleeping, allergic reactions, or fever.[2][3] Use in pregnancy or breastfeeding is generally regarded as safe.[2] When used for anemia it is recommended that pernicious anemia as a cause be ruled out first.[3] Folinic acid is a form of folic acid that does not require activation by dihydrofolate reductase to be useful to the body.[3]
Folinic acid was first made in 1945.[5] It is on the World Health Organization's List of Essential Medicines.[6]
Medical use
[edit]
Folinic acid is given following methotrexate as part of a total chemotherapeutic plan, where it may protect against bone marrow suppression or gastrointestinal mucosa inflammation. No apparent effect is seen on pre-existing methotrexate-induced nephrotoxicity.[7] Folinic acid can be taken as a pill (orally) or injected into a vein (intravenously) or muscle (intramuscularly).[8]
While not specifically an antidote for methotrexate, folinic acid may also be useful in the treatment of acute methotrexate overdose. Different dosing protocols are used, but folinic acid should be redosed until the methotrexate level is less than 5 x 10−8 M.[9]
Additionally, folinic acid is sometimes used to reduce the side effects of methotrexate in rheumatoid arthritis patients. This includes reductions in nausea, abdominal pain, abnormal liver blood tests, and mouth sores.[10]
Folinic acid is also used in combination with the chemotherapy agent 5-fluorouracil in treating colon cancer. In this case, folinic acid is not used for "rescue" purposes; rather, it enhances the effect of 5-fluorouracil by inhibiting thymidylate synthase.
Folinic acid is also sometimes used to prevent toxic effects of high doses of antimicrobial dihydrofolate reductase inhibitors such as trimethoprim and pyrimethamine, although its value for this indication has not been clearly established.[11] It may be prescribed in the treatment of toxoplasmosis retinitis, in combination with the folic acid antagonists pyrimethamine and sulfadiazine.
Folinic acid is also used in the treatment of cerebral folate deficiency, a syndrome in which the use of folic acid cannot normalize cerebrospinal fluid levels of 5-MTHF.[12]
In pyridoxine-dependent epilepsy, folinic acid may be used as additional therapy if pyridoxine or pyridoxal phosphate fails to fully control the seizures.[13]
Side effects
[edit]This section needs expansion with: with:especially about the causes of intrathecal adverse effects. You can help by adding to it. (September 2024) |
Folinic acid should not be administered intrathecally. This may produce severe adverse effects or even death.[14]
In cancer patients, rare hypersensitivity reactions to folinic acid have been described.[15]
Drug interactions
[edit]Fluorouracil: Folinic acid may increase the toxicity associated with fluorouracil if the two are administered together. Some adverse effects that have occurred, particularly in elderly patients, include severe enterocolitis, diarrhea, and dehydration.
Sulfamethoxazole-trimethoprim: A potential drug interaction exists with concomitant use of sulfamethoxazole-trimethoprim and folinic acid. Folinic acid has been shown to decrease the efficacy of sulfamethoxazole-trimethoprim in the treatment of Pneumocystis jirovecii (formerly known as Pneumocystis carinii), a common cause of pneumonia in AIDS patients.[16]
Mechanism of action
[edit]Folinic acid is a 5-formyl derivative of tetrahydrofolic acid. It is readily converted to other reduced folic acid derivatives (e.g., 5,10-methylenetetrahydrofolate, 5-methyltetrahydrofolate), thus has vitamin activity equivalent to that of folic acid. Since it does not require the action of dihydrofolate reductase for its conversion, its function as a vitamin is unaffected by inhibition of this enzyme by drugs such as methotrexate. This is the classical view of folinic acid rescue therapy. In 1980s, however, folinic acid was found to reactivate the dihydrofolate reductase itself even when methotrexate exists.
Although the mechanism is not very clear, the polyglutamylation of methotrexate and dihydrofolate in malignant cells is considered to play an important role in the selective reactivation of dihydrofolate reductase by folinic acid in normal cells.[17]
Folinic acid, therefore, allows for some purine/pyrimidine synthesis to occur in the presence of dihydrofolate reductase inhibition, so some normal DNA replication processes can proceed.
Folinic acid has dextro- and levorotary isomers. Both levoleucovorin (the levorotary isomer) and racemic folinic acid (a mixture of both isomers) have similar efficacy and tolerability.[18] Levoleucovorin was approved by the FDA in 2008.[19]
History
[edit]Folinic acid was discovered as a needed growth factor for the bacterium Leuconostoc citrovorum in 1948, by Sauberlich and Baumann.[20] This resulted in it being called "citrovorum factor," meaning citrovorum growth factor. It had an unknown structure, but was found to be a derivative of folate that had to be metabolized in the liver before it could support growth of L. citrovorum. The synthesis of citrovorum factor by liver cells in culture was eventually accomplished from pteroylglutamic acid in the presence of suitable concentrations of ascorbic acid. The simultaneous addition of sodium formate to such systems resulted in increased citrovorum factor activity in the cell-free supernatants (producing, as now known, the 5-formyl derivative), and from this method of preparation of large amounts of the factor, its structure as levo-folinic acid (5-formyl tetrahydrofolic acid) was eventually deduced.[21]
Names
[edit]Folinic acid should be distinguished from folic acid (vitamin B9). However, folinic acid is a vitamer for folic acid and has the full vitamin activity of this vitamin. Levofolinic acid and its salts are the 2S-form of the molecule. They are the only forms of the molecule that are known to be biologically active.
It is generally administered as the calcium or sodium salt (calcium folinate (INN), sodium folinate, leucovorin calcium, leucovorin sodium).
References
[edit]- ^ "Safety Data Sheet Folinic Acid (calcium salt)" (PDF). Retrieved 25 January 2018.
- ^ a b c British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 576–577. ISBN 9780857111562.
- ^ a b c d e f "Leucovorin Calcium". The American Society of Health-System Pharmacists. Archived from the original on 10 May 2017. Retrieved 8 December 2016.
- ^ Munjal YP, Sharm SK (2012). API Textbook of Medicine, Ninth Edition, Two Volume Set. JP Medical Ltd. p. 1945. ISBN 9789350250747. Archived from the original on 10 May 2017.
- ^ Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. p. 235. ISBN 9780471899792. Archived from the original on 10 May 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Therapeutic Information Resources Australia (2004). Calcium Folinate (Systemic) in AUSDI: Australian Drug Information for the Health Care Professional. Castle Hill: Therapeutic Information Resources Australia.[page needed]
- ^ McGuire BW, Sia LL, Leese PT, Gutierrez ML, Stokstad EL (January 1988). "Pharmacokinetics of leucovorin calcium after intravenous, intramuscular, and oral administration". Clinical Pharmacy. 7 (1): 52–58. PMID 3257913.
- ^ "Leucovorin" (PDF). CCO Formulary. Archived from the original (PDF) on 10 October 2008. Retrieved 7 January 2014.
- ^ Shea B, Swinden MV, Tanjong Ghogomu E, Ortiz Z, Katchamart W, Rader T, et al. (May 2013). "Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis". The Cochrane Database of Systematic Reviews. 5 (5): CD000951. doi:10.1002/14651858.CD000951.pub2. PMC 7046011. PMID 23728635.
- ^ Trubiano JA, Grayson ML (2017). "Trimethoprim and Trimethoprim–Sulfamethoxazole (Cotrimoxazole)". In Grayson ML, Cosgrove S, Crowe S, Hope W, McCarthy J, Mills J, Mouton JW, Paterson D (eds.). Kucers' the Use of Antibiotics (7th ed.). CRC Press. p. 1652. doi:10.1201/9781498747967. ISBN 9781498747967.
- ^ Gordon N (March 2009). "Cerebral folate deficiency". Developmental Medicine and Child Neurology. 51 (3): 180–182. doi:10.1111/j.1469-8749.2008.03185.x. PMID 19260931. S2CID 7373721.
- ^ Kaminiów K, Pająk M, Pająk R, Paprocka J (December 2021). "Pyridoxine-Dependent Epilepsy and Antiquitin Deficiency Resulting in Neonatal-Onset Refractory Seizures". Brain Sciences. 12 (1): 65. doi:10.3390/brainsci12010065. PMC 8773593. PMID 35053812.
- ^ Jardine LF, Ingram LC, Bleyer WA (August 1996). "Intrathecal leucovorin after intrathecal methotrexate overdose". Journal of Pediatric Hematology/Oncology. 18 (3): 302–304. doi:10.1097/00043426-199608000-00014. PMID 8689347. S2CID 43280375.
- ^ Florit-Sureda M, Conde-Estévez D, Vidal J, Montagut C (December 2016). "Hypersensitivity reaction caused by folinic acid administration: a case report and literature review". Journal of Chemotherapy. 28 (6): 500–505. doi:10.1179/1973947815Y.0000000048. hdl:10230/27696. PMID 26042586. S2CID 25420102.
- ^ Razavi B, Lund B, Allen BL, Schlesinger L (January 2002). "Failure of trimethoprim/sulfamethoxazole prophylaxis for Pneumocystis carinii pneumonia with concurrent leucovorin use". Infection. 30 (1): 41–42. doi:10.1007/s15010-001-1172-0. PMID 11876516. S2CID 35513636.
- ^ Goldman ID, Matherly LH (1987). "Biochemical factors in the selectivity of leucovorin rescue: selective inhibition of leucovorin reactivation of dihydrofolate reductase and leucovorin utilization in purine and pyrimidine biosynthesis by methotrexate and dihydrofolate polyglutamates". NCI Monographs (5): 17–26. PMID 2448654.
- ^ Kovoor PA, Karim SM, Marshall JL (October 2009). "Is levoleucovorin an alternative to racemic leucovorin? A literature review". Clinical Colorectal Cancer. 8 (4): 200–206. doi:10.3816/CCC.2009.n.034. PMID 19822510.
- ^ "FDA Approves Levoleucovorin". Drugs.com. 7 May 2008. Archived from the original on 2 July 2009. Retrieved 7 June 2009.
- ^ "A New Form of Folic Acid-The Levconostoc Citrovorum Factor". Nutrition Reviews. 8 (9): 282–284. 27 April 2009. doi:10.1111/j.1753-4887.1950.tb02478.x. PMID 14775946.
- ^ Rabinowitz, J. C., & Pricer Jr, W. E. (1956). Formimino-tetrahydrofolic acid and methenyltetrahydrofolic acid as intermediates in the formation of N10-formyltetrahydrofolic acid. Journal of the American Chemical Society, 78(21), 5702-5704.
External links
[edit]- "Leucovorin". MedlinePlus.
