Doxorubicin
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌdɒksəˈruːbɪsɪn/ |
| Trade names | Adriamycin, Caelyx,[1] Myocet,[2] others |
| Biosimilars | Zolsketil pegylated liposomal,[3] Celdoxome pegylated liposomal[4] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682221 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | intravenous, intravesical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 5% (by mouth) |
| Protein binding | 75%[8] |
| Metabolism | Liver |
| Elimination half-life | Triphasic; 12 minutes, 3.3 hours, 30 hours. Mean: 1–3 hours[8][9] |
| Excretion | Urine (5–12%), faeces (40–50%)[8] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.344 |
| Chemical and physical data | |
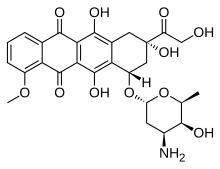
| Formula | C27H29NO11 |
| Molar mass | 543.525 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer.[10] This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia.[10] It is often used together with other chemotherapy agents.[10] Doxorubicin is given by injection into a vein.[10]
Common side effects include hair loss, bone marrow suppression, vomiting, rash, and inflammation of the mouth.[10] Other serious side effects may include allergic reactions such as anaphylaxis, heart damage, tissue damage at the site of injection, radiation recall, and treatment-related leukemia.[10] People often experience red discoloration of the urine for a few days.[10] Doxorubicin is in the anthracycline and antitumor antibiotic family of medications.[10] It works in part by interfering with the function of DNA.[11]
Doxorubicin was approved for medical use in the United States in 1974.[10] It is on the World Health Organization's List of Essential Medicines.[12][13] Versions that are pegylated and in liposomes are also available; however, they are more expensive.[13] Doxorubicin was originally made from the bacterium Streptomyces peucetius.[14]
Medical uses
[edit]In the EU doxorubicin pegylated liposomal (as Caelyx) is indicated to treat breast cancer, ovarian cancer, and AIDS-related Kaposi's sarcoma. It is indicated to treat multiple myeloma in combination with bortezomib.[1] Doxorubicin hydrochloride (as Myocet liposomal) is indicated to treat breast cancer in combination with cyclophosphamide.[2]
Doxorubicin is commonly used to treat some leukemias and lymphomas, as well as cancers of the bladder, breast, stomach, lung, ovaries, thyroid, soft tissue sarcoma as well as aggressive fibromatosis (desmoid tumor), multiple myeloma, and others.[9][15][16] Commonly used doxorubicin-containing regimens are AC (Adriamycin, cyclophosphamide), TAC (taxotere, AC), ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine), BEACOPP, CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone) and FAC (5-fluorouracil, adriamycin, cyclophosphamide).[9] Its activity is inhibited by certain antioxidant plant extracts, for example Tragia volubilis aqueous extract.[17]
Doxil (see below) is used primarily for the treatment of ovarian cancer where the disease has progressed or recurred after platinum-based chemotherapy, or for the treatment of AIDS-related Kaposi's sarcoma.[18]
Side effects
[edit]Cardiotoxicity
[edit]The most dangerous side effect of doxorubicin is dilated cardiomyopathy, leading to congestive heart failure. The rate of cardiomyopathy is dependent on its cumulative dose, with an incidence about 4% when the dose of doxorubicin is 500–550 mg/m2, 18% when the dose is 551–600 mg/m2 and 36% when the dose exceeds 600 mg/m2.[19] There are several ways in which doxorubicin is believed to cause cardiomyopathy, including oxidative stress due to iron accumulation, downregulation of genes for contractile proteins, and p53-mediated apoptosis.[19][20]
Doxorubicin-induced cardiomyopathy typically results in dilated cardiomyopathy, with all four cardiac chambers being enlarged.[21] This results in both systolic and diastolic dysfunction.[21] Eventually, heart failure can result, which carries a 50% mortality rate.[21] There is no effective treatment against established cardiomyopathy caused by the drug as of 2010.[21] The drug dexrazoxane, which is an iron chelator may be used to decrease the risk of doxorubicin's cardiotoxicity in certain cases.[22]
Other
[edit]Another common and potentially fatal complication of doxorubicin is typhlitis, an acute life-threatening inflammation of the bowel.[23] Additionally, some people may develop palmar plantar erythrodysesthesia (PPE), characterized by skin eruptions on the palms of the hand or soles of the feet, swelling, pain, and erythema.[18] Due to these side effects and its red color, doxorubicin has earned the nickname "red devil"[24][25] or "red death."[26]
Chemotherapy can cause reactivation of hepatitis B, and doxorubicin-containing regimens are no exception.[27][28]
Doxorubicin and several chemotherapeutic drugs (including cyclophosphamide) can cause a loss of skin pigmentation.[29]
Liposomal formulations
[edit]
There is a pegylated (polyethylene glycol coated) liposome-encapsulated form of doxorubicin, developed to treat Kaposi's sarcoma. The polyethylene glycol coating results in preferential concentration of doxorubicin in the skin. However, this also results in a side effect called palmar plantar erythrodysesthesia (PPE), more commonly known as hand-foot syndrome.
Following administration of this form of doxorubicin, small amounts of the drug can leak from capillaries in the palms of the hands and soles of the feet. The result of this leakage is redness, tenderness, and peeling of the skin that can be uncomfortable and even painful. In clinical testing at 50 mg/m2 dosing every four weeks, half of people developed hand-foot syndrome. The rate of this side effect limits the dose of this formulation that can be given as compared with plain doxorubicin in the same treatment regimen, thereby limiting potential substitution. Substitution would be desirable because liposome-encapsulated doxorubicin is less cardiotoxic than unencapsulated doxorubicin. This liposome-encapsulated form is also approved by the FDA for treatment of ovarian cancer and multiple myeloma.[30][31]
A non-pegylated liposomal doxorubicin, called Myocet, is approved in the European Union and in Canada for the treatment of metastatic breast cancer in combination with cyclophosphamide,[2] but it has not been approved by the FDA for use in the United States. Unlike Doxil, the Myocet liposome does not have a polyethylene glycol coating, and therefore does not result in the same rate of PPE. The minimization of this side effect may allow for one-for-one (1:1) substitution with doxorubicin in the same treatment regimen, thereby improving safety with no loss of efficacy. Like Doxil, the liposomal encapsulation of the doxorubicin limits the cardiotoxicity. In theory, by limiting the cardiotoxicity of doxorubicin through liposomal encapsulation, it can be used safely in concurrent combination with other cardiotoxic chemotherapy drugs, such as trastuzumab. There is an FDA black box warning that trastuzumab cannot be used in concurrent combination with doxorubicin, only in sequential combination. Though concurrent combination of trastuzumab and doxorubicin in clinical studies found superior tumor response, the combination resulted in unacceptable cardiotoxicity, including risk of cardiac failure manifesting as congestive heart failure (CHF). Published phase II study results have shown that Myocet, trastuzumab, and paclitaxel can safely be used concurrently without the cardiac risk, as measured by reduction in LVEF function, while still achieving superior tumor response. This finding is the basis for the ongoing phase III trial for FDA approval.[30]
Biosynthesis
[edit]Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway.
Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of Streptomyces. In contrast, only one known non-wild type species, Streptomyces peucetius subspecies cesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin.[32] This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities.[33] Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of Streptomyces can produce doxorubicin.[34] His group also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR.[35]
By 1999, they produced recombinant dox A, a cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin.[36] This was significant because it became clear that all daunorubicin-producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides.[32] Some triple mutants, that also over-expressed dox A, were able to double the yield of DXR. This is of more than academic interest, because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum.[37]
More efficient production techniques have brought the price down to $1.1 million per kg for the nonliposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps, and the yield is poor.[38] Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.
Mechanism of action
[edit]
Doxorubicin interacts with DNA by intercalation and inhibition of macromolecular biosynthesis.[11][40][41] This inhibits the progression of topoisomerase II, an enzyme which relaxes supercoils in DNA for transcription.[42] Doxorubicin stabilizes the topoisomerase II complex after it has broken the DNA chain for replication, preventing the DNA double helix from being released and thereby stopping the process of replication.[11] It may also increase quinone type free radical production, hence contributing to its cytotoxicity.[15]
The planar aromatic chromophore portion of the molecule intercalates between two base pairs of the DNA, while the six-membered daunosamine sugar sits in the minor groove and interacts with flanking base pairs immediately adjacent to the intercalation site, as evidenced by several crystal structures.[39][43]
By intercalation, doxorubicin can also induce histone eviction from transcriptionally active chromatin.[44][45] As a result, the DNA damage response, epigenome and transcriptome are deregulated in doxorubicin-exposed cells.[44]
History
[edit]
In the 1950s, an Italian research company, Farmitalia Research Laboratories, began an organized effort to find anticancer compounds from soil-based microbes. A soil sample was isolated from the area surrounding the Castel del Monte, a 13th-century castle. A new strain of Streptomyces peucetius, which produced a red pigment, was isolated, and an antibiotic from this bacterium was effective against tumors in mice. Since a group of French researchers discovered the same compound at about the same time, the two teams named the compound daunorubicin, combining the name Dauni, a pre-Roman tribe that occupied the area of Italy where the compound was isolated, with the French word for ruby, rubis, describing the color.[46][47][48] Clinical trials began in the 1960s, and the drug was successful in treating acute leukemia and lymphoma. However, by 1967, it was recognized that daunorubicin could lead to fatal cardiac toxicity.[49]
Researchers at Farmitalia soon discovered that changes in biological activity could be made by minor changes in the structure of the compound. A strain of Streptomyces was mutated using N-nitroso-N-methyl urethane, and this new strain produced a different, red-colored antibiotic. They named this new compound Adriamycin, after the Adriatic Sea, and the name was later changed to doxorubicin to conform to the established naming convention.[33] Doxorubicin showed better activity than daunorubicin against mouse tumors, and especially solid tumors. It also showed a higher therapeutic index, yet the cardiotoxicity remained.[50]
Doxorubicin and daunorubicin together can be thought of as prototype compounds for the anthracyclines. Subsequent research has led to many other anthracycline antibiotics, or analogs, and there are now over 2,000 known analogs of doxorubicin. By 1991, 553 of them had been evaluated in the screening program at the National Cancer Institute (NCI).[46] In 2016 GPX-150 was granted orphan drug designation by US FDA.[51]
Society and culture
[edit]Legal status
[edit]On 24 March 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Zolsketil pegylated liposomal, intended for the treatment of metastatic breast cancer, advanced ovarian cancer, progressive multiple myeloma and AIDS-related Kaposi's sarcoma.[52] The applicant for this medicinal product is Accord Healthcare S.L.U.[52] Zolsketil pegylated liposomal is a hybrid medicine of Adriamycin.[52] It contains the same active substance as Adriamycin, but is available in a pegylated liposomal formulation.[52] Zolsketil pegylated liposomal was approved for medical use in the European Union in May 2022.[3][53]
On 21 July 2022, the CHMP adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Celdoxome pegylated liposomal, intended for the treatment of metastatic breast cancer, advanced ovarian cancer, progressive multiple myeloma and AIDS-related Kaposi's sarcoma.[54] The applicant for this medicinal product is YES Pharmaceutical Development Services GmbH.[54] Celdoxome pegylated liposomal is a hybrid medicine of Adriamycin which has been authorized in the EU since 24 October 1979.[54] Celdoxome pegylated liposomal contains the same active substance as Adriamycin, but is available in a pegylated liposomal formulation.[54] Celdoxome pegylated liposomal was approved for medical use in the European Union in September 2022.[4]
Names
[edit]It is also known as hydroxydaunorubicin and hydroxydaunomycin.[55]
It is sold under a number of different brand names, including Adriamycin PFS, Adriamycin RDF, or Rubex.[9]
Formulations
[edit]Doxorubicin is photosensitive, and containers are often covered by an aluminum bag and/or brown wax paper to prevent light from affecting it.[9] Doxorubicin is also available in liposome-encapsulated forms as Doxil (pegylated form), Myocet (nonpegylated form), and Caelyx,[1] which are also given by intravenous injection.[9]
The FDA approved the first generic version of Doxil, made by Sun, in February 2013.[56]
Research
[edit]Combination therapy experiments with sirolimus (rapamycin) and doxorubicin have shown promise in treating Akt-positive lymphomas in mice.[57]
Further, the release of photo-activated adriamycin with the aid of nanoporous optical antenna resulted in significant anti-cancer effect in MCF-7 breast cancer cells.[58] In 2006, animal research coupling a murine monoclonal antibody with doxorubicin created an immunoconjugate that was able to eliminate HIV-1 infection in mice.[59][60]
Antimalarial activity
[edit]There is some evidence for antimalarial activity for doxorubicin and similar compounds. In 2009, a compound similar in structure to doxorubicin was found to inhibit plasmepsin II, an enzyme unique to the malarial parasite Plasmodium falciparum.[61] The pharmaceutical company GlaxoSmithKline (GSK) later identified doxorubicin in a set of compounds that inhibit parasite growth.[62]
Fluorescence
[edit]Doxorubicin is also known to be fluorescent. This has often been used to characterize doxorubicin concentrations, and has opened the possibility of using the molecule as a theranostic agent. However, there are significant limitations, as doxorubicin's fluorescence spectrum is known to depend on a variety of factors, including the pH of the environment, solvent dielectric constant and others. Doxorubicin fluorescence is quenched by binding to DNA, and shielded by micelle encapsulation. It is also known to self-quench at high concentrations. In contrast, histone binding amplifies fluorescence.[63][64]
References
[edit]- ^ a b c d "Caelyx pegylated liposomal EPAR". European Medicines Agency (EPAR). 17 September 2018. Archived from the original on 21 June 2022. Retrieved 20 June 2022.
- ^ a b c d "Myocet liposomal EPAR". European Medicines Agency. 17 September 2018. Archived from the original on 25 February 2022. Retrieved 20 June 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b c "Zolsketil pegylated liposomal EPAR". European Medicines Agency (EMA). 24 January 2022. Archived from the original on 21 June 2022. Retrieved 20 June 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b c "Celdoxome pegylated liposomal EPAR". European Medicines Agency (EMA). 20 June 2022. Retrieved 31 January 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Caelyx product information". Health Canada. 25 April 2012. Archived from the original on 21 June 2022. Retrieved 20 June 2022.
- ^ "Myocet product information". Health Canada. 25 April 2012. Retrieved 20 June 2022.
- ^ "Taro-doxorubicin liposomal product information". Health Canada. 25 April 2012. Archived from the original on 14 May 2021. Retrieved 20 June 2022.
- ^ a b c "(doxorubicin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 16 April 2014. Retrieved 15 April 2014.
- ^ a b c d e f Brayfield A, ed. (19 December 2013). "Doxorubicin". Martindale: The Complete Drug Reference. Pharmaceutical Press. Archived from the original on 28 August 2021. Retrieved 15 April 2014.
- ^ a b c d e f g h i "Doxorubicin Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 11 October 2016. Retrieved 12 January 2017.
- ^ a b c Tacar O, Sriamornsak P, Dass CR (February 2013). "Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems". The Journal of Pharmacy and Pharmacology. 65 (2): 157–170. doi:10.1111/j.2042-7158.2012.01567.x. PMID 23278683. S2CID 34737360.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ a b British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 583. ISBN 9780857111562.
- ^ Ravina E (2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 291. ISBN 9783527326693. Archived from the original on 18 September 2017.
- ^ a b Rossi S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ^ Alman B, Attia S, Baumgarten C, Benson C, Blay JY, Bonvalot S, et al. (Desmoid Tumor Working Group) (March 2020). "The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients". European Journal of Cancer. 127: 96–107. doi:10.1016/j.ejca.2019.11.013. hdl:2318/1793788. PMID 32004793. S2CID 210998595.
- ^ Bailon-Moscoso N, Coronel-Hidalgo J, Duarte-Casar R, Guamán-Ortiz LM, Figueroa JG, Romero-Benavides JC (November 2023). "Exploring the Antioxidant Potential of Tragia volubilis L.: Mitigating Chemotherapeutic Effects of Doxorubicin on Tumor Cells". Antioxidants. 12 (11): 2003. doi:10.3390/antiox12112003. ISSN 2076-3921. PMC 10669231. PMID 38001856.
- ^ a b ""DOXIL Product Information" (PDF). Archived from the original (PDF) on 21 September 2007. Retrieved 19 April 2007.
- ^ a b Chatterjee K, Zhang J, Honbo N, Karliner JS (January 2010). "Doxorubicin cardiomyopathy". Cardiology. 115 (2): 155–162. doi:10.1159/000265166. PMC 2848530. PMID 20016174.
- ^ Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, et al. (February 2014). "Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation". The Journal of Clinical Investigation. 124 (2): 617–630. doi:10.1172/JCI72931. PMID 24382354.
- ^ a b c d Chatterjee K, Zhang J, Honbo N, Karliner JS (2010). "Doxorubicin cardiomyopathy". Cardiology. 115 (2): 155–162. doi:10.1159/000265166. PMC 2848530. PMID 20016174.
- ^ "Dexrazoxane Hydrochloride Monograph for Professionals - Drugs.com". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 1 August 2018. Retrieved 1 August 2018.
- ^ Kaczmarek A, Brinkman BM, Heyndrickx L, Vandenabeele P, Krysko DV (March 2012). "Severity of doxorubicin-induced small intestinal mucositis is regulated by the TLR-2 and TLR-9 pathways". The Journal of Pathology. 226 (4): 598–608. doi:10.1002/path.3009. PMID 21960132. S2CID 206325412.[permanent dead link]
- ^ "Outpatient Oncology Drug Series: Doxorubicin is the Infamous Red Devil". Archived from the original on 21 April 2018. Retrieved 21 April 2018.
- ^ Bloch R, Bloch A. "25 Most Asked Questions". Fighting Cancer. R. A. Bloch Cancer Foundation. Archived from the original on 26 June 2007. Retrieved 28 June 2007.
- ^ Groopman JE (2007). How Doctors Think. Boston: Houghton Mifflin. p. 49. ISBN 978-0-618-61003-7.
- ^ Yeo W, Lam KC, Zee B, Chan PS, Mo FK, Ho WM, et al. (November 2004). "Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy". Annals of Oncology. 15 (11): 1661–1666. doi:10.1093/annonc/mdh430. PMID 15520068.
- ^ Dillon R, Hirschfield GM, Allison ME, Rege KP (July 2008). "Fatal reactivation of hepatitis B after chemotherapy for lymphoma". BMJ. 337: a423. doi:10.1136/bmj.39490.680498.BE. PMID 18595895. S2CID 11661945.
- ^ "Image Challenge | NEJM". Archived from the original on 16 March 2013. Retrieved 1 September 2011.
- ^ a b "Liposomal doxorubicin (Caelyx, Myocet)". Macmillan Cancer Support. 1 April 2009. Archived from the original on 29 November 2009. Retrieved 27 November 2009.
- ^ "Doxorubicin liposomal". Chemocare. Cleveland Clinic. Archived from the original on 2 January 2010. Retrieved 27 November 2009.
- ^ a b Lomovskaya N, Otten SL, Doi-Katayama Y, Fonstein L, Liu XC, Takatsu T, et al. (January 1999). "Doxorubicin overproduction in Streptomyces peucetius: cloning and characterization of the dnrU ketoreductase and dnrV genes and the doxA cytochrome P-450 hydroxylase gene". Journal of Bacteriology. 181 (1): 305–318. doi:10.1128/JB.181.1.305-318.1999. PMC 103563. PMID 9864344.
- ^ a b Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C, et al. (November 1969). "Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius". Biotechnology and Bioengineering. 11 (6): 1101–1110. doi:10.1002/bit.260110607. PMID 5365804. S2CID 21897153.
- ^ Grimm A, Madduri K, Ali A, Hutchinson CR (December 1994). "Characterization of the Streptomyces peucetius ATCC 29050 genes encoding doxorubicin polyketide synthase". Gene. 151 (1–2): 1–10. doi:10.1016/0378-1119(94)90625-4. PMID 7828855.
- ^ Dickens ML, Strohl WR (June 1996). "Isolation and characterization of a gene from Streptomyces sp. strain C5 that confers the ability to convert daunomycin to doxorubicin on Streptomyces lividans TK24". Journal of Bacteriology. 178 (11): 3389–3395. doi:10.1128/jb.178.11.3389-3395.1996. PMC 178102. PMID 8655530.
- ^ Walczak RJ, Dickens ML, Priestley ND, Strohl WR (January 1999). "Purification, properties, and characterization of recombinant Streptomyces sp. strain C5 DoxA, a cytochrome P-450 catalyzing multiple steps in doxorubicin biosynthesis". Journal of Bacteriology. 181 (1): 298–304. doi:10.1128/JB.181.1.298-304.1999. PMC 103562. PMID 9864343.
- ^ Hutchinson CR, Colombo AL (July 1999). "Genetic engineering of doxorubicin production in Streptomyces peucetius: a review". Journal of Industrial Microbiology & Biotechnology. 23 (1): 647–652. doi:10.1038/sj.jim.2900673. PMID 10455495. S2CID 27337697.
- ^ Lown JW (November 1993). "Anthracycline and anthraquinone anticancer agents: current status and recent developments". Pharmacology & Therapeutics. 60 (2): 185–214. doi:10.1016/0163-7258(93)90006-Y. PMID 8022857.
- ^ a b Frederick CA, Williams LD, Ughetto G, van der Marel GA, van Boom JH, Rich A, et al. (March 1990). "Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin". Biochemistry. 29 (10): 2538–2549. doi:10.1021/bi00462a016. PMID 2334681. Crystal structure is available for download as a PDB Archived 14 January 2008 at the Wayback Machine file.
- ^ Fornari FA, Randolph JK, Yalowich JC, Ritke MK, Gewirtz DA (April 1994). "Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells". Molecular Pharmacology. 45 (4): 649–656. PMID 8183243.
- ^ Momparler RL, Karon M, Siegel SE, Avila F (August 1976). "Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells". Cancer Research. 36 (8): 2891–2895. PMID 1277199. Archived from the original on 5 February 2009.
- ^ Pommier Y, Leo E, Zhang H, Marchand C (May 2010). "DNA topoisomerases and their poisoning by anticancer and antibacterial drugs". Chemistry & Biology. 17 (5): 421–433. doi:10.1016/j.chembiol.2010.04.012. PMC 7316379. PMID 20534341.
- ^ Pigram WJ, Fuller W, Hamilton LD (January 1972). "Stereochemistry of intercalation: interaction of daunomycin with DNA". Nature. 235 (53): 17–19. doi:10.1038/newbio235017a0. PMID 4502404.
- ^ a b Pang B, Qiao X, Janssen L, Velds A, Groothuis T, Kerkhoven R, et al. (2013). "Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin". Nature Communications. 4 (5): 1908. Bibcode:2013NatCo...4.1908P. doi:10.1038/ncomms2921. PMC 3674280. PMID 23715267.
- ^ Pang B, de Jong J, Qiao X, Wessels LF, Neefjes J (July 2015). "Chemical profiling of the genome with anti-cancer drugs defines target specificities". Nature Chemical Biology. 11 (7): 472–480. doi:10.1038/nchembio.1811. PMID 25961671.
- ^ a b Weiss RB (December 1992). "The anthracyclines: will we ever find a better doxorubicin?". Seminars in Oncology. 19 (6): 670–686. PMID 1462166.
- ^ Baruffa G (1966). "Clinical trials in Plasmodium falciparum malaria with a long-acting sulphonamide". Transactions of the Royal Society of Tropical Medicine and Hygiene. 60 (2): 222–224. doi:10.1016/0035-9203(66)90030-7. PMID 5332105.
- ^ Per prior citation, the first publication: Camerino B, Palamidessi G (1960). "Derivati della parazina II. Sulfonamdopir". Gazz Chim Ital (in Italian). 90: 1802–1815.
- ^ Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA (March 1967). "Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia". Cancer. 20 (3): 333–353. doi:10.1002/1097-0142(1967)20:3<333::AID-CNCR2820200302>3.0.CO;2-K. PMID 4290058. S2CID 19272219.
- ^ Di Marco A, Gaetani M, Scarpinato B (February 1969). "Adriamycin (NSC-123,127): a new antibiotic with antitumor activity". Cancer Chemotherapy Reports. 53 (1): 33–37. PMID 5772652.
- ^ Investigational Sarcoma Drug GPX-150 Gets Orphan Drug Designation. 2016 Archived 24 January 2016 at the Wayback Machine
- ^ a b c d "Zolsketil pegylated liposomal: Pending EC decision". European Medicines Agency (EMA). 24 March 2022. Archived from the original on 29 March 2022. Retrieved 29 March 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Zolsketil Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ a b c d "Celdoxome pegylated liposomal: Pending EC decision". European Medicines Agency (EMA). 22 July 2022. Archived from the original on 28 July 2022. Retrieved 30 July 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Doxorubicin: MedlinePlus Drug Information". medlineplus.gov. Archived from the original on 13 July 2020. Retrieved 12 July 2020.
- ^ "FDA approval of generic version of cancer drug Doxil is expected to help resolve shortage" (Press release). U.S. Food and Drug Administration (FDA). 4 February 2013. Archived from the original on 28 February 2014. Retrieved 22 February 2014.
- ^ Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. (March 2004). "Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy". Nature. 428 (6980): 332–337. Bibcode:2004Natur.428..332W. doi:10.1038/nature02369. PMID 15029198. S2CID 4426215.
- ^ Saha T, Mondal J, Khiste S, Lusic H, Hu ZW, Jayabalan R, et al. (September 2021). "Nanotherapeutic approaches to overcome distinct drug resistance barriers in models of breast cancer". Nanophotonics. 10 (12): 3063–3073. Bibcode:2021Nanop..10..142S. doi:10.1515/nanoph-2021-0142. PMC 8478290. PMID 34589378.
- ^ Johansson S, Goldenberg DM, Griffiths GL, Wahren B, Hinkula J (October 2006). "Elimination of HIV-1 infection by treatment with a doxorubicin-conjugated anti-envelope antibody". AIDS. 20 (15): 1911–1915. doi:10.1097/01.aids.0000247111.58961.60. PMID 16988511. S2CID 42286690.
- ^ Mitsuyasu R (May 2013). "Curing HIV: lessons from cancer therapy". Current Opinion in HIV and AIDS. 8 (3): 224–229. doi:10.1097/COH.0b013e32835ef0a1. PMC 3789644. PMID 23454863.
- ^ Friedman R, Caflisch A (August 2009). "Discovery of plasmepsin inhibitors by fragment-based docking and consensus scoring". ChemMedChem. 4 (8): 1317–1326. doi:10.1002/cmdc.200900078. PMID 19472268. S2CID 14642593. Archived from the original on 5 January 2013. Retrieved 28 May 2010.
- ^ Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, et al. (May 2010). "Thousands of chemical starting points for antimalarial lead identification". Nature. 465 (7296): 305–310. Bibcode:2010Natur.465..305G. doi:10.1038/nature09107. PMID 20485427. S2CID 1143258.
- ^ Karukstis KK, Thompson EH, Whiles JA, Rosenfeld RJ (July 1998). "Deciphering the fluorescence signature of daunomycin and doxorubicin". Biophysical Chemistry. 73 (3): 249–263. doi:10.1016/s0301-4622(98)00150-1. PMID 9700924.
- ^ Mohan P, Rapoport N (December 2010). "Doxorubicin as a molecular nanotheranostic agent: effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking". Molecular Pharmaceutics. 7 (6): 1959–1973. doi:10.1021/mp100269f. PMC 2997862. PMID 20957997.
External links
[edit] Media related to Doxorubicin at Wikimedia Commons
Media related to Doxorubicin at Wikimedia Commons- "Doxorubicin". Drug Information Portal. U.S. National Library of Medicine.
- "Doxorubicin hydrochloride". Drug Information Portal. U.S. National Library of Medicine.
