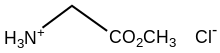
Glycine methyl ester hydrochloride
Appearance
| |
| Names | |
|---|---|
| IUPAC name
Methyl glycinate hydrochloride
| |
| Systematic IUPAC name
Methyl 2-aminoacetate hydrochloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.024.672 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H8ClNO2 | |
| Molar mass | 125.55 g·mol−1 |
| Appearance | white solid |
| Melting point | 175–176 °C (347–349 °F; 448–449 K) |
| Hazards | |
| GHS labelling:[1] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glycine methyl ester hydrochloride is the organic compound with the formula [CH3O2CCH2NH3]Cl. A white, water-soluble solid, it is the hydrochloride of the methyl ester of the amino acid glycine.
Synthesis and reactions
[edit]Glycine methyl ester hydrochloride can be prepared by treatment of glycine with 2 equivalents of trimethylsilyl chloride, followed by the addition of methanol.[2][3]
Upon treatment with base, the salt converts to glycine methyl ester.[4]
Glycine methyl ester (and other esters of glycine) are not shelf-stable, tending to polymerize when stored at room temperature[4] or convert to diketopiperazine. The hydrochloride is shelf-stable.
References
[edit]- ^ "Glycine methyl ester hydrochloride". pubchem.ncbi.nlm.nih.gov. Retrieved 24 January 2022.
- ^ Li, Jiabo; Sha, Yaowu (2008). "A Convenient Synthesis of Amino Acid Methyl Esters". Molecules. 13 (5): 1111–1119. doi:10.3390/molecules13051111. PMC 6245331. PMID 18560331.
- ^ White, James D.; Kranemann, Christian L.; Kuntiyong, Punlop (2002). "4-Methoxycarbonyl-2-methyl-1,3-oxazole". Org. Synth. 79: 244. doi:10.15227/orgsyn.079.0244.
- ^ a b Myers, Andrew G.; Gleason, James L. (1999). "Asymmetric Synthesis of α-Amino Acids by the Alkylation of Pseudoephedrine Glycinamide: L-Allylglycine and N-BOC-l-Allylglycine". Organic Syntheses. 76: 57. doi:10.15227/orgsyn.076.0057.
