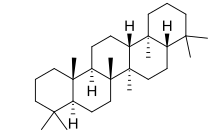
Gammacerane
Appearance
| |
| Names | |
|---|---|
| IUPAC name
Gammacerane[1]
| |
| Systematic IUPAC name
(4aS,6aR,6bR,8aS,12aS,12bR,14aR,14bS)-4,4,6a,6b,9,9,12a,14b-Octamethyldocosahydropicene | |
| Identifiers | |
3D model (JSmol)
|
|
| 2562711 | |
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H52 | |
| Molar mass | 412.746 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Gammacerane is a pentacyclic triterpene compound with the formula C30H52 and five six-membered rings. Its derivatives include tetrahymanol(gammaceran-3β-ol)and so on. After millions of years of diagenesis, these derivatives became gammacerane can be used as biomarkers in petroleum to study the origin of petroleum.[2]
See also
[edit]References
[edit]- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1535. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Ten Haven HL, Rohmer M, Rullkötter J, Bisseret P (November 1989). "Tetrahymanol, the most likely precursor of gammacerane, occurs ubiquitously in marine sediments". Geochimica et Cosmochimica Acta. 53 (11): 3073–3079. Bibcode:1989GeCoA..53.3073T. doi:10.1016/0016-7037(89)90186-5.
