Ferroxyl indicator solution
Appearance
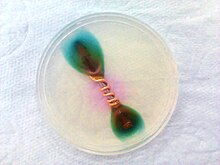
Ferroxyl indicator, or rust indicator, is a solution containing potassium hexacyanoferrate(III), phenolphthalein and sodium chloride. It turns blue in the presence of Fe2+ ions, and pink in the presence of hydroxide (OH-) ions. It can be used to detect metal oxidation, and is often used to detect rusting in various situations.
Formula
[edit]It can be prepared by dissolving 10g sodium chloride and 1g potassium hexacyanoferrate(III) in distilled water, adding 10 cm3 phenolphthalein indicator, then making up to 500 cm3 with distilled water.[1]
References
[edit]- ^ "SALTERS ADVANCED CHEMISTRY A2 TECHNICIANS MANUAL" (PDF). benjamin-mills.com. p. 52.
