Protein kinase R
Protein kinase RNA-activated also known as protein kinase R (PKR), interferon-induced, double-stranded RNA-activated protein kinase, or eukaryotic translation initiation factor 2-alpha kinase 2 (EIF2AK2) is an enzyme that in humans is encoded by the EIF2AK2 gene on chromosome 2.[5][6] PKR is a serine/tyrosine kinase that is 551 amino acids long.[7]
PKR is inducible by various mechanisms of stress and protects against viral infections.[8] It also has a role in several signaling pathways.[9][10]
Mechanism of action
[edit]Protein kinase-R is activated by double-stranded RNA (dsRNA), introduced to the cells by a viral infection.[9] In situations of viral infection, the dsRNA created by viral replication and gene expression binds to the N-terminal domain, activating the protein.[9] PKR activation via dsRNA is length dependent, requiring the dsRNA to be 30 bp in length to bind to PKR molecules.[9] However, excess dsRNA can diminish activation of PKR.[9] Binding to dsRNA is believed to activate PKR by inducing dimerization of the kinase domains and subsequent auto-phosphorylation reactions.[9] It is not yet established whether PKR activates in cis, with a protomer's activation loop reaching into its own catalytic site, or in trans, with the activation loop being phosphorylated in a face to face geometry by a conjugate protomer.[11] PKR can also be activated by the protein PACT via phosphorylation of S287 on its M3 domain.[12] The promoter region of PKR has interferon-stimulated response elements to which Type I interferons (IFN) bind to induce the transcription of PKR genes.[12][13] Some research suggests that PKR can be stimulated by heat shock proteins, heparin, growth factors, bacterial infection, pro-inflammatory cytokines, reactive oxygen species, DNA damage, mechanical stress, and excess nutrient intake.[12]
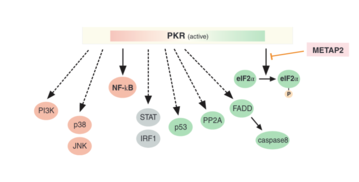
Once active, PKR is able to phosphorylate the eukaryotic translation initiation factor eIF2α.[12] This inhibits further cellular mRNA translation, thereby preventing viral protein synthesis.[10] Overall, this leads to apoptosis of virally infected cells to prevent further viral spread. PKR can also induce apoptosis in bacterial infection by responding to LPS and proinflammatory cytokines.[10] Apoptosis can also occur via PKR activation of the FADD and caspase signaling pathway.[13]
PKR also has pro-inflammatory functions, as it can mediate the activation of the transcription factor NF-kB, by phosphorylating its inhibitory subunit, IkB.[13] This leads to the expression of adhesion molecules and transcription factors that activate them, which induce inflammation responses such as the secretion of pro-inflammatory cytokines.[12] PKR also activates several mitogen-activated protein kinases (MAPK) to lead to inflammation.[13]
To balance the effects of apoptosis and inflammation, PKR has regulatory functions. Active PKR is also able to activate tumor suppressor PP2A which regulates the cell cycle and the metabolism.[14] There is also evidence that PKR is autophagic as a regulatory mechanism.[13]
PKR stress pathway
[edit]PKR is in the center of cellular response to different stress signals such as pathogens, lack of nutrients, cytokines, irradiation, mechanical stress, or ER stress.[12] The PKR pathway leads to a stress response through activation of other stress pathways such as JNK, p38, NFkB, PP2A and phosphorylation of eIF2α.[10] ER stress caused by excess of unfolded proteins leads to inflammatory responses.[15] PKR contributes to this response by interacting with several inflammatory kinases such as IKK, JNK, ElF2α, insulin receptors and others.[15] This metabolically activated inflammatory complex is called metabolic inflammasome or metaflammasome.[16][17] Via the JNK signaling pathway, PKR also plays a role in insulin resistance, diabetes, and obesity by phosphorylating IRS1.[18] Inhibiting PKR in mice led to lower inflammation in adipose tissues, increased sensitivity to insulin, and amelioration of diabetic symptoms.[18] PKR also participates in the mitochondrial unfolded protein response (UPRmt).[19] Here, PKR is induced via the transcription factor AP-1 and activated independently of PACT.[19] In this context, PKR has been shown to be relevant to intestinal inflammation.[19]
Viral defense
[edit]Viruses have developed many mechanisms to counteract the PKR mechanism. It may be done by Decoy dsRNA, degradation, hiding of viral dsRNA, dimerization block, dephosphorylation of substrate or by a pseudosubstrate.
For instance, Epstein–Barr virus (EBV) uses the gene EBER1 to produce decoy dsRNA. This leads to cancers such as Burkitt's lymphoma, Hodgkin's disease, nasopharyngeal carcinoma and various leukemias.
| Defense type | Virus | Molecule |
|---|---|---|
| Decoy dsRNA | Adenovirus | VAI RNA |
| Epstein–Barr virus | EBER | |
| HIV | TAR | |
| PKR degradation | Poliovirus | 2Apro |
| Hide viral dsRNA | Vaccinia virus | E3L |
| Reovirus | σ3 | |
| Influenza virus | NS1 | |
| Dimerization block | Influenza virus | p58IPK |
| Hepatitis C virus | NS5A | |
| Pseudosubstrate | Vaccinia virus | K3L |
| HIV | Tat | |
| Dephosphorylation of substrate | Herpes simplex virus | ICP34.5 |
Memory and learning
[edit]PKR knockout mice or inhibition of PKR in mice enhances memory and learning.[20]
Neuronal degeneration disease
[edit]First report in 2002 has been shown that immunohistochemical marker for phosphorylated PKR and eIF2α was displayed positively in degenerating neurons in the hippocampus and the frontal cortex of patients with Alzheimer's disease (AD), suggesting the link between PKR and AD. Additionally, many of these neurons were also immunostained with an antibody for phosphorylated Tau protein.[21] Activated PKR was specifically found in the cytoplasm and nucleus, as well as co-localized with neuronal apoptotic markers.[22] Further studies have assessed the levels of PKR in blood and cerebrospinal fluid (CSF) of AD patients and controls. The result of an analysis of the concentrations of total and phosphorylated PKR (pPKR) in peripheral blood mononuclear cells (PBMCs) in 23 AD patients and 19 control individuals showed statistically significant increased levels of the ratio of phosphorylated PKR/PKR in AD patients compared with controls.[23] Assessments of CSF biomarkers, such as Aβ1-42, Aβ1-40, Tau, and phosphorylated Tau at threonine 181, have been a validated use in clinical research and in routine practice to determine whether patients have CSF abnormalities and AD brain lesions. A study found that "total PKR and pPKR concentrations were elevated in AD and amnestic mild cognitive impairment subjects with a pPKR value (optical density units) discriminating AD patients from control subjects with a sensitivity of 91.1% and a specificity of 94.3%. Among AD patients, total PKR and pPKR levels correlate with CSF p181tau levels. Some AD patients with normal CSF Aß, T-tau, or p181tau levels had abnormal total PKR and pPKR levels".[24] It was concluded that the PKR-eIF2α pro-apoptotic pathway could be involved in neuronal degeneration that leads to various neuropathological lesions as a function of neuronal susceptibility.
PKR and beta amyloid
Activation of PKR can cause accumulation of amyloid β-peptide (Aβ) via de-repression of BACE1 (β-site APP Cleaving Enzyme) expression in Alzheimer Disease patients.[25] Normally, the 5′ untranslated region (5′ UTR) in the BACE1 promoter would fundamentally inhibit the expression of BACE1 gene. However, BACE1 expression can be activated by phosphorylation of eIF2a, which reverses the inhibitory effect exerted by BACE1 5′ UTR. Phosphorylation of eIF2a is triggered by activation of PKR. Viral infection such as herpes simplex virus (HSV) or oxidative stress can both increase BACE1 expression through activation of PKR-eIF2a pathway.[26]
In addition, the increased activity of BACE1 could also lead to β-cleaved carboxy-terminal fragment of β-Amyloid precursor protein (APP-βCTF) induced dysfunction of endosomes in AD.[27] Endosomes are highly active β-Amyloid precursor protein (APP) processing sites, and endosome abnormalities are associated with upregulated expression of early endosomal regulator, Rab5. These are the earliest known disease-specific neuronal response in AD. Increased activity of BACE1 leads to synthesis of the APP-βCTF. An elevated level of βCTF then causes Rab5 overactivation. βCTF recruits APPL1 to rab5 endosomes, where it stabilizes active GTP-Rab5, leading to pathologically accelerated endocytosis, endosome swelling and selectively impaired axonal transport of Rab5 endosomes.
PKR and Tau phosphorylation
It is reported earlier that phosphorylated PKR could co-localize with phosphorylated Tau protein in affected neurons.[28][21] A protein phosphatase-2A inhibitor (PP2A inhibitor) – okadaic acid (OA) – is known to increase tau phosphorylation, Aβ deposition and neuronal death. It is studied that OA also induces PKR phosphorylation and thus, eIF2a phosphorylation. eIF2a phosphorylation then induces activation of transcription factor 4 (ATF4), which induces apoptosis and nuclear translocation, contributing to neuronal death.[29]
Glycogen synthase kinase 3β (GSK-3β) is responsible for tau phosphorylation and controls several cellular functions including apoptosis. Another study demonstrated that tunicamycin or Aβ treatment can induce PKR activation in human neuroblastoma cells and can trigger GSK3β activation, as well as tau phosphorylation. They found that in AD brains, both activated PKR and GSK3β co-localize with phosphorylated tau in neurons. In SH-SY5Y cell cultures, tunicamycin and Aβ(1-42) activate PKR, which then can modulate GSK-3β activation and induce tau phosphorylation, apoptosis. All these processes are attenuated by PKR inhibitors or PKR siRNA. PKR could represent a crucial signaling point relaying stress signals to neuronal pathways by interacting with transcription factor or indirectly controlling GSK3β activation, leading to cellular degeneration in AD.[30]
Fetal alcohol syndrome
[edit]PKR also mediates ethanol-induced protein synthesis inhibition and apoptosis which is linked to fetal alcohol syndrome.[31]
Interactions
[edit]Protein kinase R has been shown to interact with:
- ASK1,[32]
- DNAJC3,[33]
- ILF3,[34][35][36][37]
- METAP2,[38]
- P53,[39]
- PPP1CA,[40]
- PRKRA,[41][42]
- STAT1,[43][44] and
- TARBP2.[45][46]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000055332 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000024079 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Entrez Gene: EIF2AK2 eukaryotic translation initiation factor 2-alpha kinase 2".
- ^ Feng GS, Chong K, Kumar A, Williams BR (June 1992). "Identification of double-stranded RNA-binding domains in the interferon-induced double-stranded RNA-activated p68 kinase". Proceedings of the National Academy of Sciences of the United States of America. 89 (12): 5447–5451. Bibcode:1992PNAS...89.5447F. doi:10.1073/pnas.89.12.5447. PMC 49309. PMID 1351683.
- ^ Gupta P, Taiyab A, Hassan MI (January 2021). Donev R (ed.). "Emerging role of protein kinases in diabetes mellitus: From mechanism to therapy". Advances in Protein Chemistry and Structural Biology. Protein Kinases in Drug Discovery. 124. Academic Press: 47–85. doi:10.1016/bs.apcsb.2020.11.001. ISBN 9780323853132. PMID 33632470. S2CID 229608384.
- ^ Bou-Nader C, Gordon JM, Henderson FE, Zhang J (May 2019). "The search for a PKR code-differential regulation of protein kinase R activity by diverse RNA and protein regulators". RNA. 25 (5): 539–556. doi:10.1261/rna.070169.118. PMC 6467004. PMID 30770398.
- ^ a b c d e f Matz KM, Guzman RM, Goodman AG (2019-01-01). Vanpouille-Box C, Galluzzi L (eds.). "The Role of Nucleic Acid Sensing in Controlling Microbial and Autoimmune Disorders". International Review of Cell and Molecular Biology. Nucleic Acid Sensing and Immunity - Part B. 345. Academic Press: 35–136. doi:10.1016/bs.ircmb.2018.08.002. ISBN 9780128159811. PMC 6445394. PMID 30904196.
- ^ a b c d Lee YS, Kunkeaw N, Lee YS (March 2020). "Protein kinase R and its cellular regulators in cancer: An active player or a surveillant?". Wiley Interdisciplinary Reviews. RNA. 11 (2): e1558. doi:10.1002/wrna.1558. PMID 31231984. S2CID 195327674.
- ^ Mayo CB, Erlandsen H, Mouser DJ, Feinstein AG, Robinson VL, May ER, Cole JL (July 2019). "Structural Basis of Protein Kinase R Autophosphorylation". Biochemistry. 58 (27): 2967–2977. doi:10.1021/acs.biochem.9b00161. PMC 6615999. PMID 31246429.
- ^ a b c d e f Gal-Ben-Ari S, Barrera I, Ehrlich M, Rosenblum K (2019). "PKR: A Kinase to Remember". Frontiers in Molecular Neuroscience. 11: 480. doi:10.3389/fnmol.2018.00480. PMC 6333748. PMID 30686999.
- ^ a b c d e Smyth R, Sun J (2021). "Protein Kinase R in Bacterial Infections: Friend or Foe?". Frontiers in Immunology. 12: 702142. doi:10.3389/fimmu.2021.702142. PMC 8297547. PMID 34305942.
- ^ Cheng X, Byrne M, Brown KD, Konopleva MY, Kornblau SM, Bennett RL, May WS (September 2015). "PKR inhibits the DNA damage response, and is associated with poor survival in AML and accelerated leukemia in NHD13 mice". Blood. 126 (13): 1585–1594. doi:10.1182/blood-2015-03-635227. PMC 4582335. PMID 26202421.
- ^ a b Lee ES, Yoon CH, Kim YS, Bae YS (September 2007). "The double-strand RNA-dependent protein kinase PKR plays a significant role in a sustained ER stress-induced apoptosis". FEBS Letters. 581 (22): 4325–4332. doi:10.1016/j.febslet.2007.08.001. PMID 17716668. S2CID 45865684.
- ^ García MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M (December 2006). "Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action". Microbiology and Molecular Biology Reviews. 70 (4): 1032–1060. doi:10.1128/MMBR.00027-06. PMC 1698511. PMID 17158706.
- ^ Hotamisligil GS (March 2010). "Endoplasmic reticulum stress and the inflammatory basis of metabolic disease". Cell. 140 (6): 900–917. doi:10.1016/j.cell.2010.02.034. PMC 2887297. PMID 20303879.
- ^ a b Mu X, Ahmad S, Hur S (2016-01-01). Alt FW (ed.). Endogenous Retroelements and the Host Innate Immune Sensors. Advances in Immunology. Vol. 132. Academic Press. pp. 47–69. doi:10.1016/bs.ai.2016.07.001. ISBN 9780128047972. PMC 5135014. PMID 27769507.
- ^ a b c Rath E, Berger E, Messlik A, Nunes T, Liu B, Kim SC, et al. (September 2012). "Induction of dsRNA-activated protein kinase links mitochondrial unfolded protein response to the pathogenesis of intestinal inflammation". Gut. 61 (9): 1269–1278. doi:10.1136/gutjnl-2011-300767. PMC 4514769. PMID 21997551.
- ^ Zhu PJ, Huang W, Kalikulov D, Yoo JW, Placzek AN, Stoica L, et al. (December 2011). "Suppression of PKR promotes network excitability and enhanced cognition by interferon-γ-mediated disinhibition". Cell. 147 (6): 1384–1396. doi:10.1016/j.cell.2011.11.029. PMC 3569515. PMID 22153080.
- Hilary Roberts (December 19, 2011). "Discovery could lead memory-enhancing pill out of realm of science fiction". Canada.
- ^ a b Chang RC, Wong AK, Ng HK, Hugon J (December 2002). "Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer's disease". NeuroReport. 13 (18): 2429–2432. doi:10.1097/00001756-200212200-00011. PMID 12499843. S2CID 84266563.
- ^ Page G, Rioux Bilan A, Ingrand S, Lafay-Chebassier C, Pain S, Perault Pochat MC, et al. (2006). "Activated double-stranded RNA-dependent protein kinase and neuronal death in models of Alzheimer's disease". Neuroscience. 139 (4): 1343–1354. doi:10.1016/j.neuroscience.2006.01.047. PMID 16581193. S2CID 36700744.
- ^ Paccalin M, Pain-Barc S, Pluchon C, Paul C, Besson MN, Carret-Rebillat AS, et al. (2006). "Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer's disease". Dementia and Geriatric Cognitive Disorders. 22 (4): 320–326. doi:10.1159/000095562. PMID 16954686. S2CID 45647507.
- ^ Mouton-Liger F, Paquet C, Dumurgier J, Lapalus P, Gray F, Laplanche JL, Hugon J (May 2012). "Increased cerebrospinal fluid levels of double-stranded RNA-dependant protein kinase in Alzheimer's disease". Biological Psychiatry. 71 (9): 829–835. doi:10.1016/j.biopsych.2011.11.031. PMID 22281122. S2CID 21131086.
- ^ Ill-Raga G, Palomer E, Wozniak MA, Ramos-Fernández E, Bosch-Morató M, Tajes M, et al. (2011-06-28). "Activation of PKR causes amyloid ß-peptide accumulation via de-repression of BACE1 expression". PLOS ONE. 6 (6): e21456. Bibcode:2011PLoSO...621456I. doi:10.1371/journal.pone.0021456. PMC 3125189. PMID 21738672.
- ^ Mouton-Liger F, Paquet C, Dumurgier J, Bouras C, Pradier L, Gray F, Hugon J (June 2012). "Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2α pathway". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1822 (6): 885–896. doi:10.1016/j.bbadis.2012.01.009. PMID 22306812.
- ^ Kim S, Sato Y, Mohan PS, Peterhoff C, Pensalfini A, Rigoglioso A, et al. (May 2016). "Evidence that the rab5 effector APPL1 mediates APP-βCTF-induced dysfunction of endosomes in Down syndrome and Alzheimer's disease". Molecular Psychiatry. 21 (5): 707–716. doi:10.1038/mp.2015.97. PMC 4721948. PMID 26194181.
- ^ Peel AL, Bredesen DE (October 2003). "Activation of the cell stress kinase PKR in Alzheimer's disease and human amyloid precursor protein transgenic mice". Neurobiology of Disease. 14 (1): 52–62. doi:10.1016/S0969-9961(03)00086-X. PMID 13678666. S2CID 13109874.
- ^ Kim SM, Yoon SY, Choi JE, Park JS, Choi JM, Nguyen T, Kim DH (September 2010). "Activation of eukaryotic initiation factor-2 α-kinases in okadaic acid-treated neurons". Neuroscience. 169 (4): 1831–1839. doi:10.1016/j.neuroscience.2010.06.016. PMID 20600673. S2CID 207248721.
- ^ Bose A, Mouton-Liger F, Paquet C, Mazot P, Vigny M, Gray F, Hugon J (March 2011). "Modulation of tau phosphorylation by the kinase PKR: implications in Alzheimer's disease". Brain Pathology. 21 (2): 189–200. doi:10.1111/j.1750-3639.2010.00437.x. PMC 8094269. PMID 21029237. S2CID 39517621.
- ^ Chen G, Ma C, Bower KA, Ke Z, Luo J (June 2006). "Interaction between RAX and PKR modulates the effect of ethanol on protein synthesis and survival of neurons". The Journal of Biological Chemistry. 281 (23): 15909–15915. doi:10.1074/jbc.M600612200. PMID 16574643.
- ^ Takizawa T, Tatematsu C, Nakanishi Y (December 2002). "Double-stranded RNA-activated protein kinase interacts with apoptosis signal-regulating kinase 1. Implications for apoptosis signaling pathways". European Journal of Biochemistry. 269 (24): 6126–6132. doi:10.1046/j.1432-1033.2002.03325.x. PMID 12473108.
- ^ Polyak SJ, Tang N, Wambach M, Barber GN, Katze MG (January 1996). "The P58 cellular inhibitor complexes with the interferon-induced, double-stranded RNA-dependent protein kinase, PKR, to regulate its autophosphorylation and activity". The Journal of Biological Chemistry. 271 (3): 1702–1707. doi:10.1074/jbc.271.3.1702. PMID 8576172.
- ^ Saunders LR, Perkins DJ, Balachandran S, Michaels R, Ford R, Mayeda A, Barber GN (August 2001). "Characterization of two evolutionarily conserved, alternatively spliced nuclear phosphoproteins, NFAR-1 and -2, that function in mRNA processing and interact with the double-stranded RNA-dependent protein kinase, PKR". The Journal of Biological Chemistry. 276 (34): 32300–32312. doi:10.1074/jbc.M104207200. PMID 11438536.
- ^ Langland JO, Kao PN, Jacobs BL (May 1999). "Nuclear factor-90 of activated T-cells: A double-stranded RNA-binding protein and substrate for the double-stranded RNA-dependent protein kinase, PKR". Biochemistry. 38 (19): 6361–6368. doi:10.1021/bi982410u. PMID 10320367.
- ^ Parker LM, Fierro-Monti I, Mathews MB (August 2001). "Nuclear factor 90 is a substrate and regulator of the eukaryotic initiation factor 2 kinase double-stranded RNA-activated protein kinase". The Journal of Biological Chemistry. 276 (35): 32522–32530. doi:10.1074/jbc.M104408200. PMID 11438540.
- ^ Patel RC, Vestal DJ, Xu Z, Bandyopadhyay S, Guo W, Erme SM, et al. (July 1999). "DRBP76, a double-stranded RNA-binding nuclear protein, is phosphorylated by the interferon-induced protein kinase, PKR". The Journal of Biological Chemistry. 274 (29): 20432–20437. doi:10.1074/jbc.274.29.20432. PMID 10400669.
- ^ Gil J, Esteban M, Roth D (December 2000). "In vivo regulation of the dsRNA-dependent protein kinase PKR by the cellular glycoprotein p67". Biochemistry. 39 (51): 16016–16025. doi:10.1021/bi001754t. PMID 11123929.
- ^ Cuddihy AR, Wong AH, Tam NW, Li S, Koromilas AE (April 1999). "The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro". Oncogene. 18 (17): 2690–2702. doi:10.1038/sj.onc.1202620. PMID 10348343.
- ^ Tan SL, Tareen SU, Melville MW, Blakely CM, Katze MG (September 2002). "The direct binding of the catalytic subunit of protein phosphatase 1 to the PKR protein kinase is necessary but not sufficient for inactivation and disruption of enzyme dimer formation". The Journal of Biological Chemistry. 277 (39): 36109–36117. doi:10.1074/jbc.M205109200. PMID 12138106.
- ^ Huang X, Hutchins B, Patel RC (August 2002). "The C-terminal, third conserved motif of the protein activator PACT plays an essential role in the activation of double-stranded-RNA-dependent protein kinase (PKR)". The Biochemical Journal. 366 (Pt 1): 175–186. doi:10.1042/BJ20020204. PMC 1222748. PMID 11985496.
- ^ Patel RC, Sen GC (August 1998). "PACT, a protein activator of the interferon-induced protein kinase, PKR". The EMBO Journal. 17 (15): 4379–4390. doi:10.1093/emboj/17.15.4379. PMC 1170771. PMID 9687506.
- ^ Wong AH, Tam NW, Yang YL, Cuddihy AR, Li S, Kirchhoff S, et al. (March 1997). "Physical association between STAT1 and the interferon-inducible protein kinase PKR and implications for interferon and double-stranded RNA signaling pathways". The EMBO Journal. 16 (6): 1291–1304. doi:10.1093/emboj/16.6.1291. PMC 1169727. PMID 9135145.
- ^ Wong AH, Durbin JE, Li S, Dever TE, Decker T, Koromilas AE (April 2001). "Enhanced antiviral and antiproliferative properties of a STAT1 mutant unable to interact with the protein kinase PKR". The Journal of Biological Chemistry. 276 (17): 13727–13737. doi:10.1074/jbc.M011240200. PMID 11278865.
- ^ Cosentino GP, Venkatesan S, Serluca FC, Green SR, Mathews MB, Sonenberg N (October 1995). "Double-stranded-RNA-dependent protein kinase and TAR RNA-binding protein form homo- and heterodimers in vivo". Proceedings of the National Academy of Sciences of the United States of America. 92 (21): 9445–9449. Bibcode:1995PNAS...92.9445C. doi:10.1073/pnas.92.21.9445. PMC 40818. PMID 7568151.
- ^ Daher A, Longuet M, Dorin D, Bois F, Segeral E, Bannwarth S, et al. (September 2001). "Two dimerization domains in the trans-activation response RNA-binding protein (TRBP) individually reverse the protein kinase R inhibition of HIV-1 long terminal repeat expression". The Journal of Biological Chemistry. 276 (36): 33899–33905. doi:10.1074/jbc.M103584200. PMID 11438532.
Further reading
[edit]- Williams BR (November 1999). "PKR; a sentinel kinase for cellular stress". Oncogene. 18 (45): 6112–6120. doi:10.1038/sj.onc.1203127. PMID 10557102.
- García MA, Meurs EF, Esteban M (2007). "The dsRNA protein kinase PKR: virus and cell control". Biochimie. 89 (6–7): 799–811. doi:10.1016/j.biochi.2007.03.001. PMID 17451862.
- Thomis DC, Doohan JP, Samuel CE (May 1992). "Mechanism of interferon action: cDNA structure, expression, and regulation of the interferon-induced, RNA-dependent P1/eIF-2 alpha protein kinase from human cells". Virology. 188 (1): 33–46. doi:10.1016/0042-6822(92)90732-5. PMID 1373553.
- McCormack SJ, Thomis DC, Samuel CE (May 1992). "Mechanism of interferon action: identification of a RNA binding domain within the N-terminal region of the human RNA-dependent P1/eIF-2 alpha protein kinase". Virology. 188 (1): 47–56. doi:10.1016/0042-6822(92)90733-6. PMID 1373554.
- Mellor H, Proud CG (July 1991). "A synthetic peptide substrate for initiation factor-2 kinases". Biochemical and Biophysical Research Communications. 178 (2): 430–437. doi:10.1016/0006-291X(91)90125-Q. PMID 1677563.
- Meurs E, Chong K, Galabru J, Thomas NS, Kerr IM, Williams BR, Hovanessian AG (July 1990). "Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon". Cell. 62 (2): 379–390. doi:10.1016/0092-8674(90)90374-N. PMID 1695551. S2CID 20477995.
- Silverman RH, Sengupta DN (1991). "Translational regulation by HIV leader RNA, TAT, and interferon-inducible enzymes". Journal of Experimental Pathology. 5 (2): 69–77. PMID 1708818.
- Roy S, Katze MG, Parkin NT, Edery I, Hovanessian AG, Sonenberg N (March 1990). "Control of the interferon-induced 68-kilodalton protein kinase by the HIV-1 tat gene product". Science. 247 (4947): 1216–1219. Bibcode:1990Sci...247.1216R. doi:10.1126/science.2180064. PMID 2180064.
- McMillan NA, Chun RF, Siderovski DP, Galabru J, Toone WM, Samuel CE, et al. (November 1995). "HIV-1 Tat directly interacts with the interferon-induced, double-stranded RNA-dependent kinase, PKR". Virology. 213 (2): 413–424. doi:10.1006/viro.1995.0014. PMID 7491766.
- Barber GN, Edelhoff S, Katze MG, Disteche CM (June 1993). "Chromosomal assignment of the interferon-inducible double-stranded RNA-dependent protein kinase (PRKR) to human chromosome 2p21-p22 and mouse chromosome 17 E2". Genomics. 16 (3): 765–767. doi:10.1006/geno.1993.1262. PMID 7686883.
- Squire J, Meurs EF, Chong KL, McMillan NA, Hovanessian AG, Williams BR (June 1993). "Localization of the human interferon-induced, ds-RNA activated p68 kinase gene (PRKR) to chromosome 2p21-p22". Genomics. 16 (3): 768–770. doi:10.1006/geno.1993.1263. PMID 7686884.
- Prigmore E, Ahmed S, Best A, Kozma R, Manser E, Segal AW, Lim L (May 1995). "A 68-kDa kinase and NADPH oxidase component p67phox are targets for Cdc42Hs and Rac1 in neutrophils". The Journal of Biological Chemistry. 270 (18): 10717–10722. doi:10.1074/jbc.270.18.10717. PMID 7738010.
- Barber GN, Wambach M, Wong ML, Dever TE, Hinnebusch AG, Katze MG (May 1993). "Translational regulation by the interferon-induced double-stranded-RNA-activated 68-kDa protein kinase". Proceedings of the National Academy of Sciences of the United States of America. 90 (10): 4621–4625. Bibcode:1993PNAS...90.4621B. doi:10.1073/pnas.90.10.4621. PMC 46564. PMID 8099444.
- Chen ZJ, Parent L, Maniatis T (March 1996). "Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity". Cell. 84 (6): 853–862. doi:10.1016/S0092-8674(00)81064-8. PMID 8601309. S2CID 112412.
- Kuhen KL, Shen X, Carlisle ER, Richardson AL, Weier HU, Tanaka H, Samuel CE (August 1996). "Structural organization of the human gene (PKR) encoding an interferon-inducible RNA-dependent protein kinase (PKR) and differences from its mouse homolog". Genomics. 36 (1): 197–201. doi:10.1006/geno.1996.0446. PMID 8812437.
- Taylor DR, Lee SB, Romano PR, Marshak DR, Hinnebusch AG, Esteban M, Mathews MB (November 1996). "Autophosphorylation sites participate in the activation of the double-stranded-RNA-activated protein kinase PKR". Molecular and Cellular Biology. 16 (11): 6295–6302. doi:10.1128/mcb.16.11.6295. PMC 231632. PMID 8887659.
- Kuhen KL, Shen X, Samuel CE (October 1996). "Mechanism of interferon action sequence of the human interferon-inducible RNA-dependent protein kinase (PKR) deduced from genomic clones". Gene. 178 (1–2): 191–193. doi:10.1016/0378-1119(96)00314-9. PMID 8921913.