Dixanthogen disulfide
Appearance
(Redirected from Dixanthogen)
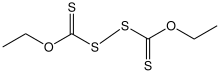
Structure of diethyl dixanthogen disulfide.
Dixanthogen disulfides are a class of organosulfur compounds with the formula (ROC(S)S)2. Usually yellow solids, they are the product of the oxidation of xanthate salts.[1] A common derivative is diethyl dixanthogen disulfide. Diisopropyl dixanthogen disulfide is commercially available. They are structurally related to thiuram disulfides.
Uses and reactions
[edit]Diethyl dixanthogen disulfide is a component for froth flotations used, inter alia, for the separation of sulfide minerals like pyrrhotite. Diisopropyl dixanthogen disulfide is a reagent in the synthesis of sulfur heterocycles.[2]
Dialkoxy dixanthogen disulfides undergo desulfurization by cyanide to give bis(alkoxythiocarbonyl)sulfides:[3]
- (ROC(S)S)2 + CN− → (ROC(S))2S + SCN−
Dixanthogens are also ectoparasiticides.
References
[edit]- ^ Schroll, Alayne L.; Barany, George (1986). "Novel Symmetrical and Mixed Carbamoyl and Aminopolysulfanes by Reactions of (Alkoxydichloromethyl)polysulfanyl Substrates with N-Methylaniline". The Journal of Organic Chemistry. 51 (10): 1866–1881. doi:10.1021/jo00360a039.
- ^ Gareau, Yves; Beauchemin, André (1998). "Free Radical Reaction of Diisopropyl Xanthogen Disulfide with Unsaturated Systems". Heterocycles. 48 (10): 2003. doi:10.3987/COM-98-8230.
- ^ Tobón, Yeny A.; Castellano, Eduardo E.; Piro, Oscar E.; Della Védova, Carlos O.; Romano, Rosana M. (2009). "Spectroscopic and structural studies of bis[isopropoxy(thiocarbonyl)]sulfide, [(CH3)2CHOC(S)]2S". Journal of Molecular Structure. 930 (1–3): 43–48. Bibcode:2009JMoSt.930...43T. doi:10.1016/j.molstruc.2009.04.033.

