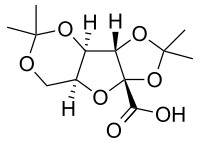
Diprogulic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3aS,3bR,7aS,8aR)-2,2,5,5-Tetramethyltetrahydro-2H,5H,8aH-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxole-8a-carboxylic acid | |
| Other names
Dikegulac acid; Diacetone-2-ketogulonic acid; Oxogulonic acid diacetonide; Sodium dikegulac; Sodium diprogulate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.038.484 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H18O7 | |
| Molar mass | 274.269 g·mol−1 |
| Appearance | White/colorless solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diprogulic acid (also known as dikegulac) is a precursor used in commercial ascorbic acid production.[1] In agriculture, its sodium salt, dikegulac sodium, is used as a plant growth regulator, primarily used as a branching agent. When it is taken up by a plant, dikegulac sodium is translocated to its apical meristems, where it inhibits DNA synthesis.[2] This suppresses apical dominance in the plant and can stimulate lateral branching, resulting in a bushier growth habit.[3] Dikegulac sodium is sometimes used to inhibit fruiting and flowering.[4]
Application methods
[edit]Dikegulac sodium can be applied as a foliar spray[5] or a trunk injection.[6]
Phytotoxicity
[edit]Dikegulac sodium application sometimes causes phytotoxicity. Symptoms include chlorosis and stunted growth.[3][7] When higher concentrations are applied, there is a greater risk that these adverse effects will persist, leading to crop loss.[3]
Reversing growth inhibition
[edit]Gibberellins can be applied to fight undesired growth inhibition following dikegulac sodium application, but success can be limited.[2]
Regulations
[edit]Plant protection products containing dikegulac were phased out in the European Union after the European Commission decided in 2002 not to include the chemical in Annex I to Directive 91/414/EEC.[8]
Dikegulac sodium is approved for EPA registration in the United States.[9]
Commercial formulations
[edit]Commercial formulations available in the United States include Atrimmec,[10] Augeo,[5] and Pinscher.[6]
Ginkgo Gate
[edit]In Fall 2008, Washington, D.C.'s Urban Forestry Administration failed to suppress the fruiting of thousands of female Ginkgo biloba trees by injecting them with the dikegulac sodium product Pinscher.
Ginkgo biloba is a dioecious plant. Because the females are well known for their foul smelling fruit, the non-fruiting males are usually recommended for landscape use. However, these city trees were installed before Ginkgo saplings could easily be sexed, so many planted were female.
The Urban Forestry Administration had previously sprayed the trees with chlorpropham to prevent fruiting, but their success had been limited. When the dikegulac sodium injection was unsuccessful, the fruit matured and dropped from the trees. Some referred to the failure as "Ginkgo Gate".[11][12]
References
[edit]- ^ Elks, J.; Ganellin, C.R. (2014). The Dictionary of Drugs: Chemical Data, Structures and Bibliographies. Springer. p. 632. ISBN 978-1-4757-2085-3.
- ^ a b Bocion, P.F.; De Silva, W.H. (1976). "Some Effects of Dikegulac on the Physiology of Whole Plants and Tissues; Interactions with Plant Hormones". In Pilet, Paul-Emile (ed.). Plant Growth Regulation (Proceedings in Life Sciences). International Conference on Plant Growth Substances, 9th. Springer Science & Business Media. pp. 189–198. ISBN 978-3-642-66589-9.
- ^ a b c Arzee, Tova; Langenauer, Haviva; Gressel, J. (1977). "Effects of Dikegulac, a New Growth Regulator, on Apical Growth and Development of Three Compositae". Botanical Gazette. 138 (1): 18–28. doi:10.1086/336891. ISSN 0006-8071. JSTOR 2473627. S2CID 84707696.
- ^ Banko, Thomas; Stefani, Marcia (1995). "Growth Regulators for Management of Fruit Production on American Sweetgum". Journal of Arboriculture. 21 (2): 88–89. ISSN 0278-5226 – via International Society of Arboriculture.
- ^ a b "Augeo Plant Growth Regulator" (PDF). OHP, Inc. Retrieved 2017-01-07.
- ^ a b "Pinscher | ArborSystems". www.arborsystems.com. Retrieved 2017-01-08.
- ^ Sun, Youping; Bi, Guihong; Niu, Genhua; Perez, Christina (2015-06-01). "Foliar Application of Dikegulac Sodium Increases Branching of 'Merritt's Supreme' Bigleaf Hydrangea". HortTechnology. 25 (3): 306–312. doi:10.21273/HORTTECH.25.3.306. ISSN 1063-0198.
- ^ "Commission Regulation (EC) No 2076/2002". eur-lex.europa.eu. Retrieved 2017-01-09.
- ^ "Reregistration Eligibility Decision for Dikegulac sodium". nephis.epa.gov. Retrieved 2017-01-09.
- ^ "Atrimmec Plant Growth Regulator". www.gordonsprofessional.com. Retrieved 2017-01-08.
- ^ Drahl, Carmen (2009-12-14). "Ginkgogate: The Stench Of Scandal". Chemical & Engineering News. Vol. 87, no. 50. p. 48. ISSN 0009-2347. Retrieved 2017-01-09.
- ^ Fahrenthold, David A. (2008-12-13). "Ginkgo-Lined D.C., Capital of the U.S., and Now P.U." The Washington Post. ISSN 0190-8286. Retrieved 2017-01-09.
