Dioxygenyl
| |
| Names | |
|---|---|
| IUPAC name
Dioxygenyl
| |
| Identifiers | |
| ChEBI | |
| ChemSpider | |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The dioxygenyl ion, O+
2, is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of +1/2. It is formally derived from oxygen by the removal of an electron:
- O2 → O+
2 + e−
The energy change for this process is called the ionization energy of the oxygen molecule. Relative to most molecules, this ionization energy is very high at 1175 kJ/mol.[1] As a result, the scope of the chemistry of O+
2 is quite limited, acting mainly as a 1-electron oxidiser.[2]
Structure and molecular properties
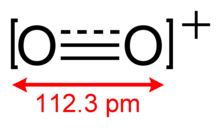
[edit]O+
2 has a bond order of 2.5, and a bond length of 112.3 pm in solid O2[AsF6].[3] It is isoelectronic with nitric oxide and is paramagnetic.[4] The bond energy is 625.1 kJ mol−1 and the stretching frequency is 1858 cm−1,[5] both of which are high relative to most of the molecules.
Synthesis
[edit]Neil Bartlett demonstrated that dioxygenyl hexafluoroplatinate (O2PtF6), containing the dioxygenyl cation, can be prepared at room temperature by direct reaction of oxygen gas (O2) with platinum hexafluoride (PtF6):[6]
- O2 + PtF6 → [O
2]+
[PtF
6]−
The compound can also be prepared from a mixture of fluorine and oxygen gases in the presence of a platinum sponge at 450 °C, and from oxygen difluoride (OF
2) above 400 °C:[6]
- 6 OF
2 + 2 Pt → 2 [O
2][PtF
6] + O
2
At lower temperatures (around 350 °C), platinum tetrafluoride is produced instead of dioxygenyl hexafluoroplatinate.[6] Dioxygenyl hexafluoroplatinate played a pivotal role in the discovery of noble gas compounds. The observation that PtF6 is a powerful enough oxidising agent to oxidise O2 (which has a first ionization potential of 12.2 eV) led Bartlett to reason that it should also be able to oxidise xenon (first ionization potential 12.13 eV). His subsequent investigation yielded the first compound of a noble gas, xenon hexafluoroplatinate.[7]
O+
2 is also found in similar compounds of the form O2MF6, where M is arsenic (As), antimony (Sb),[8] gold (Au),[9] niobium (Nb), ruthenium (Ru), rhenium (Re), rhodium (Rh),[10] vanadium (V),[11] or phosphorus (P).[12] Other forms are also attested, including O2GeF5 and (O2)2SnF6.[11]
The tetrafluoroborate and hexafluorophosphate salts may be prepared by the reaction of dioxygen difluoride with boron trifluoride or phosphorus pentafluoride at −126 °C:[12]
- 2 O2F2 + 2 BF3 → 2 O2BF4 + F2
- 2 O2F2 + 2 PF5 → 2 O2PF6 + F2
These compounds rapidly decompose at room temperature:
- 2 O2BF4 → 2 O2 + F2 + 2 BF3
- 2 O2PF6 → 2 O2 + F2 + 2 PF5
Some compounds including O2Sn2F9, O2Sn2F9·0.9HF, O2GeF5·HF, and O2[Hg(HF)]4(SbF6)9 can be made by ultraviolet irradiation of oxygen and fluorine dissolved in anhydrous hydrogen fluoride with a metal oxide.[13]
All attempts to prepare O+
2 with chloro anions like [O
2]+
[SbCl
6]−
met with failure.
Reactions
[edit]The reaction of O2BF4 with xenon at 173 K (−100 °C) produces a white solid believed to be F–Xe–BF2, containing an unusual xenon-boron bond:[14]
- 2 O2BF4 + 2 Xe → 2 O2 + F2 + 2 FXeBF2
The dioxygenyl salts O2BF4 and O2AsF6 react with carbon monoxide to give oxalyl fluoride, C2O2F2, in high yield.[15]
References
[edit]- ^ Michael Clugston; Rosalind Flemming (2000). Advanced Chemistry, Oxford University Press, ISBN 0-19-914633-0, ISBN 978-0-19-914633-8, p. 355.
- ^ Foote, Christopher S.; Valentine, Joan S. (1995). Active oxygen in chemistry. Joel F. Liebman, A. Greenberg. Springer. ISBN 0-412-03441-7.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. p. 616
- ^ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ^ J. Shamir; J. Binenboym; H. H. Claassen (1968). "The vibrational frequency of the O2+ cation". J. Am. Chem. Soc. 90 (22): 6223–6224. doi:10.1021/ja01024a054.
- ^ a b c Bartlett, Neil; Lohmann, D. H. (1962). "Fluorides of the Noble Metals. Part II. Dioxygenyl hexafluoroplatinate(V), [O
2]+
[PtF
6]−
". J. Chem. Soc. 115: 5253–5261. doi:10.1039/jr9620005253. - ^ Bartlett, Neil (1962). "Xenon hexafluoroplatinate(V), Xe+
[PtF
6]−
". Proc. Chem. Soc.: 197–236. doi:10.1039/PS9620000197. - ^ Young, A. R.; Hirata, T.; Morrow, S. I. (1964). "The Preparation of Dioxygenyl Salts from Dioxygen Difluoride". J. Am. Chem. Soc. 86 (1): 20–22. doi:10.1021/ja01055a006.
- ^ Nakajima, Tsuyoshi (1995). Fluorine-carbon and fluoride-carbon materials: chemistry, physics, and applications. CRC Press. ISBN 0-8247-9286-6.
- ^ Vasile, Michael J.; Falconer, Warren E. (1975). "Vapour transport of dioxygenyl salts". J. Chem. Soc., Dalton Trans. 1975 (4): 316–318. doi:10.1039/DT9750000316.
- ^ a b Holleman, Arnold F.; Wiberg, Egon (2001). Inorganic chemistry. Academic Press. p. 475. ISBN 0-12-352651-5.
- ^ a b Solomon, Irvine J.; Brabets, Robert I.; Uenishi, Roy K.; Keith, James N.; McDonough, John M. (1964). "New Dioxygenyl Compounds". Inorg. Chem. 3 (3): 457. doi:10.1021/ic50013a036.
- ^ Mazej, Zoran; Goreshnik, Evgeny (2020-02-03). "Syntheses of Dioxygenyl Salts by Photochemical Reactions in Liquid Anhydrous Hydrogen Fluoride: X-ray Crystal Structures of α- and β-O2Sn2F9, O2Sn2F9·0.9HF, O2GeF5·HF, and O2[Hg(HF)]4(SbF6)9". Inorganic Chemistry. 59 (3): 2092–2103. doi:10.1021/acs.inorgchem.9b03518. ISSN 0020-1669. PMC 7307900. PMID 31942804.
- ^ Goetschel, C. T.; Loos, K. R. (1972). "Reaction of xenon with dioxygenyl tetrafluoroborate. Preparation of FXe-BF2". Journal of the American Chemical Society. 94 (9): 3018–3021. doi:10.1021/ja00764a022.
- ^ Pernice, H.; Willner, H.; Eujen, R. (2001). "The reaction of dioxygenyl salts with 13
CO Formation of F13
C(O)13
C(O)F". Journal of Fluorine Chemistry. 112 (2): 277–590. doi:10.1016/S0022-1139(01)00512-7.
External links
[edit] Media related to Dioxygenyl at Wikimedia Commons
Media related to Dioxygenyl at Wikimedia Commons
