Difluorosilane
| |||
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| F2H2Si | |||
| Molar mass | 68.098 g·mol−1 | ||
| Appearance | colourless gas | ||
| Melting point | −122 °C (−188 °F; 151 K) | ||
| Boiling point | −77.8 °C (−108.0 °F; 195.3 K) | ||
| Thermochemistry[1] | |||
Std molar
entropy (S⦵298) |
262.12 J/mol•K | ||
Std enthalpy of
formation (ΔfH⦵298) |
-790.78 kJ/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
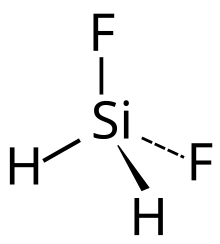
Difluorosilane is a gaseous chemical compound with formula SiH2F2. It can be considered as a derivative of silane with two hydrogen atoms replaced with fluorine.
Production
[edit]Difluorosilane can be made by fluorinating dichlorosilane with antimony trifluoride. [2] [3]
- 3 SiH2Cl2 + 2 SbF3 → 3 SiH2F2 + 2 SbCl3
Some is also made in a reaction of silicon tetrafluoride with hydrogen
- SiF4 + 2 H2 → SiH2F2 + 2 HF
Traces of difluorosilane are made when coal is burnt.[4]
Properties
[edit]Difluorosilane is a gas with boiling point −77.8 °C, and a freezing point of −122 °C. It has no colour. The silicon–fluorine bond length in difluorosilane is 1.358 Å which is greater than that in fluorosilane but less than the length in trifluorosilane.[5]
Reactions
[edit]In an electric discharge, hydrogen atoms are preferentially removed from the molecule and SiHF2SiHF2 is formed along with hydrogen.[3]
- 2 SiH2F2 → SiHF2SiHF2 + H2
At elevated temperatures, difluorosilane can disproportionate by swapping hydrogen and fluorine atoms between molecules to form fluorosilane and trifluorosilane.[5]
Use
[edit]Difluorosilane is used in dental varnish in order to prevent tooth cavities.[6]
Difluorosilane is also used in chemical vapour deposition to deposit silicon nitride films.
References
[edit]- ^ Chase, M. W. (1998). "NIST-JANAF Themochemical Tables, Fourth Edition": 1–1951.
{{cite journal}}: Cite journal requires|journal=(help) - ^ 郝润蓉 等. 无机化学丛书 第三卷 碳 硅 锗分族. 科学出版社, 1998. pp 178. 2.3 卤代硅烷
- ^ a b Addison, C. C. (1973). Inorganic Chemistry of the Main-Group Elements. Royal Society of Chemistry. p. 188. ISBN 9780851867526.
- ^ Kruszewski, Łukasz; Fabiańska, Monika J.; Ciesielczuk, Justyna; Segit, Tomasz; Orłowski, Ryszard; Motyliński, Rafał; Kusy, Danuta; Moszumańska, Izabela (November 2018). "First multi-tool exploration of a gas-condensate-pyrolysate system from the environment of burning coal mine heaps: An in situ FTIR and laboratory GC and PXRD study based on Upper Silesian materials". Science of the Total Environment. 640–641: 1044–1071. Bibcode:2018ScTEn.640.1044K. doi:10.1016/j.scitotenv.2018.05.319. PMID 30021271. S2CID 51703425.
- ^ a b Ebsworth, E. A. V. (2013). Volatile Silicon Compounds: International Series of Monographs on Inorganic Chemistry. Elsevier. pp. 54–56. ISBN 9781483180557.
- ^ Brambilla, Eugenio (2001). "Fluoride – Is It Capable of Fighting Old and New Dental Diseases?". Caries Research. 35 (1): 6–9. doi:10.1159/000049101. PMID 11359049. S2CID 24969435.


