Dengue fever
| Dengue fever | |
|---|---|
| Other names | Dengue, breakbone fever[1][2] |
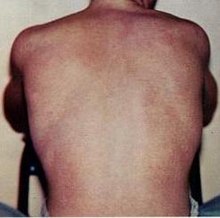 | |
| Typical rash seen in dengue fever | |
| Pronunciation | |
| Specialty | Infectious disease |
| Symptoms | Fever, headache, muscle and joint pain, rash. Can be severe, mild or asymptomatic[1][2] |
| Complications | Bleeding, low levels of blood platelets, dangerously low blood pressure[2] |
| Usual onset | 3–14 days after exposure[2] |
| Duration | 2–7 days[1] |
| Causes | Dengue virus by Aedes mosquitos[1] |
| Diagnostic method | Detecting antibodies to the virus or its RNA[2] |
| Differential diagnosis | Malaria, yellow fever, viral hepatitis, leptospirosis[5] |
| Prevention | Dengue fever vaccine, decreasing mosquito exposure[1][6] |
| Treatment | Supportive care, intravenous fluids, blood transfusions[2] |
| Frequency | 5 million per year (2023)[7] |
| Deaths | 5,000 per year (2023)[7] |
Dengue fever is a mosquito-borne disease caused by dengue virus, prevalent in tropical and subtropical areas. It is frequently asymptomatic; if symptoms appear they typically begin 3 to 14 days after infection. These may include a high fever, headache, vomiting, muscle and joint pains, and a characteristic skin itching and skin rash. Recovery generally takes two to seven days. In a small proportion of cases, the disease develops into severe dengue (previously known as dengue hemorrhagic fever or dengue shock syndrome)[8] with bleeding, low levels of blood platelets, blood plasma leakage, and dangerously low blood pressure.[1][2]
Dengue virus has four confirmed serotypes; infection with one type usually gives lifelong immunity to that type, but only short-term immunity to the others. Subsequent infection with a different type increases the risk of severe complications.[9] The symptoms of dengue resemble many other diseases including malaria, influenza, and Zika.[10] Blood tests are available to confirm the diagnosis including detecting viral RNA, or antibodies to the virus.[11]
There is no specific treatment for dengue fever. In mild cases, treatment is focused on treating pain symptoms. Severe cases of dengue require hospitalisation; treatment of acute dengue is supportive and includes giving fluid either by mouth or intravenously.[1][2]
Dengue is spread by several species of female mosquitoes of the Aedes genus, principally Aedes aegypti.[1] Infection can be prevented by mosquito elimination and the prevention of bites.[12] Two types of dengue vaccine have been approved and are commercially available. Dengvaxia became available in 2016 but it is only recommended to prevent re-infection in individuals who have been previously infected.[13] The second vaccine, Qdenga, became available in 2022 and is suitable for adults, adolescents and children from four years of age.[14]
The earliest descriptions of a dengue outbreak date from 1779; its viral cause and spread were understood by the early 20th century.[15] Already endemic in more than one hundred countries, dengue is spreading from tropical and subtropical regions to the Iberian Peninsula and the southern states of the US, partly attributed to climate change.[7][16] It is classified as a neglected tropical disease.[17] During 2023, more than 5 million infections were reported, with more than 5,000 dengue-related deaths.[7] As most cases are asymptomatic or mild, the actual numbers of dengue cases and deaths are under-reported.[7]
Signs and symptoms
[edit]Typically, people infected with dengue virus are asymptomatic (80%) or have only mild symptoms such as an uncomplicated fever.[18][19] Others have more severe illness (5%), and in a small proportion it is life-threatening.[18][19] The incubation period (time between exposure and onset of symptoms) ranges from 3 to 14 days, but most often it is 4 to 7 days.[20]
The characteristic symptoms of mild dengue are sudden-onset fever, headache (typically located behind the eyes), muscle and joint pains, nausea, vomiting, swollen glands and a rash.[1][12] If this progresses to severe dengue the symptoms are severe abdominal pain, persistent vomiting, rapid breathing, bleeding gums or nose, fatigue, restlessness, blood in vomit or stool, extreme thirst, pale and cold skin, and feelings of weakness.[1]
Clinical course
[edit]The course of infection is divided into three phases: febrile, critical, and recovery.[21]
The febrile phase involves high fever (40 °C/104 °F), and is associated with generalized pain and a headache; this usually lasts two to seven days.[22][1] There may also be nausea, vomiting, a rash, and pains in the muscle and joints.[1]
Most people recover within a week or so. In about 5% of cases, symptoms worsen and can become life-threatening. This is called severe dengue (formerly called dengue hemorrhagic fever or dengue shock syndrome).[21][23] Severe dengue can lead to shock, internal bleeding, organ failure and even death.[24] Warning signs include severe stomach pain, vomiting, difficulty breathing, and blood in the nose, gums, vomit or stools.[24]
During this period, there is leakage of plasma from the blood vessels, together with a reduction in platelets.[24] This may result in fluid accumulation in the chest and abdominal cavity as well as depletion of fluid from the circulation and decreased blood supply to vital organs.[23]
The recovery phase usually lasts two to three days.[23] The improvement is often striking, and can be accompanied with severe itching and a slow heart rate.[23]
Complications and sequelae
[edit]Complications following severe dengue include fatigue, somnolence, headache, concentration impairment and memory impairment.[21][25] A pregnant woman who develops dengue is at higher risk of miscarriage, low birth weight, and premature birth.[26]
Children and older individuals are at a risk of developing complications from dengue fever compared to other age groups; young children typically suffer from more intense symptoms. Concurrent infections with tropical diseases[27] like Zika[28] virus can worsen symptoms and make recovery more challenging.[citation needed]
Cause
[edit]Virology
[edit]
Dengue virus (DENV) is an RNA virus of the family Flaviviridae; genus Flavivirus. Other members of the same genus include yellow fever virus, West Nile virus, and Zika virus. Dengue virus genome (genetic material) contains about 11,000 nucleotide bases, which code for the three structural protein molecules (C, prM and E) that form the virus particle and seven other protein molecules that are required for replication of the virus.[29][30] There are four confirmed strains of the virus, called serotypes, referred to as DENV-1, DENV-2, DENV-3 and DENV-4. The distinctions between the serotypes are based on their antigenicity.[31]
Transmission
[edit]
Dengue virus is most frequently transmitted by the bite of mosquitos in the Aedes genus, particularly A. aegypti.[32] They prefer to feed at dusk and dawn,[33] but they may bite and thus spread infection at any time of day.[34] Other Aedes species that may transmit the disease include A. albopictus, A. polynesiensis and A. scutellaris. Humans are the primary host of the virus,[35] but it also circulates in nonhuman primates, and can infect other mammals.[36][37] An infection can be acquired via a single bite.[38]
For 2 to 10 days after becoming newly infected, a person's bloodstream will contain a high level of virus particles (the viremic period). A female mosquito that takes a blood meal from the infected host then propagates the virus in the cells lining its gut.[39] Over the next few days, the virus spreads to other tissues including the mosquito's salivary glands and is released into its saliva. Next time the mosquito feeds, the infectious saliva will be injected into the bloodstream of its victim, thus spreading the disease.[40] The virus seems to have no detrimental effect on the mosquito, which remains infected for life.[20]
Dengue can also be transmitted via infected blood products and through organ donation.[1] Vertical transmission (from mother to child) during pregnancy or at birth has been reported.[41]
Risk factors
[edit]The principal risk for infection with dengue is the bite of an infected mosquito.[42] This is more probable in areas where the disease is endemic, especially where there is high population density, poor sanitation, and standing water where mosquitoes can breed.[42] It can be mitigated by taking steps to avoid bites such as by wearing clothing that fully covers the skin, using mosquito netting while resting, and/or the application of insect repellent (DEET being the most effective).[38]
Chronic diseases – such as asthma, sickle cell anemia, and diabetes mellitus – increase the risk of developing a severe form of the disease.[43] Other risk factors for severe disease include female sex, and high body mass index,[21][30] Infection with one serotype is thought to produce lifelong immunity to that type, but only short-term protection against the other three.[22] Subsequent re-infection with a different serotype increases the risk of severe complications due to phenomenon known as antibody-dependent enhancement (ADE).[9][44]
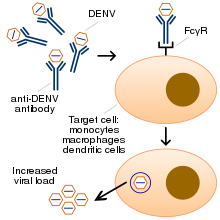
The exact mechanism of ADE is not fully understood.[45] It appears that ADE occurs when the antibodies generated during an immune response recognize and bind to a pathogen, but they fail to neutralize it. Instead, the antibody-virus complex has an enhanced ability to bind to the Fcγ receptors of the target immune cells, enabling the virus to infect the cell and reproduce itself.[44][46]
Mechanism of infection
[edit]When a mosquito carrying dengue virus bites a person, the virus enters the skin together with the mosquito's saliva. The virus infects nearby skin cells called keratinocytes, as well as specialized immune cell located in the skin, called a Langerhans cells.[47] The Langerhans cells migrate to the lymph nodes, where the infection spreads to white blood cells, and reproduces inside the cells while they move throughout the body.[48]
The white blood cells respond by producing several signaling proteins, such as cytokines and interferons, which are responsible for many of the symptoms, such as the fever, the flu-like symptoms, and the severe pains. In severe infection, the virus production inside the body is greatly increased, and many more organs (such as the liver and the bone marrow) can be affected. Fluid from the bloodstream leaks through the wall of small blood vessels into body cavities due to increased capillary permeability. As a result, blood volume decreases, and the blood pressure becomes so low that it cannot supply sufficient blood to vital organs. The spread of the virus to the bone marrow leads to reduced numbers of platelets, which are necessary for effective blood clotting; this increases the risk of bleeding, the other major complication of dengue fever.[48]
Prevention
[edit]Vector control
[edit]
The principal risk for infection with dengue is the bite of an infected mosquito.[1] This is more probable in areas where the disease is endemic, especially where there is high population density, poor sanitation, and standing water where mosquitoes can breed.[42] It can be mitigated by taking steps to avoid bites such as by wearing clothing that fully covers the skin, using mosquito netting while resting, and/or the application of insect repellent (DEET being the most effective);[49] it is also advisable to treat clothing, nets and tents with 0.5% permethrin.[50]
Protection of the home can be achieved with door and window screens, by using air conditioning, and by regularly emptying and cleaning all receptacles both indoors and outdoors which may accumulate water (such as buckets, planters, pools or trashcans).[50]
The primary method of controlling A. aegypti is by eliminating its habitats. This is done by eliminating open sources of water, or if this is not possible, by adding insecticides or biological control agents to these areas. Generalized spraying with organophosphate or pyrethroid insecticides, while sometimes done, is not thought to be effective.[51] Reducing open collections of water through environmental modification is the preferred method of control, given the concerns of negative health effects from insecticides and greater logistical difficulties with control agents. Ideally, mosquito control would be a community activity, e.g. when all members of a community clear blocked gutters and street drains and keep their yards free of containers with standing water.[52] If residences have direct water connections this eliminates the need for wells or street pumps and water-carrying containers.[52]
Vaccine
[edit]As of March 2024, there are two vaccines to protect against dengue infection; Dengvaxia and Qdenga.[53]

Dengvaxia (formerly CYD-TDV) became available in 2015, and is approved for use in the US, EU and in some Asian and Latin American countries.[54] It is an attenuated virus, is suitable for individuals aged 6–45 years and protects against all four serotypes of dengue.[55] Due to safety concerns about antibody-dependent enhancement (ADE), it should only be given to individuals who have previously been infected with dengue, in order to protect them from reinfection.[56] It is given subcutaneously as three doses at six month intervals.[57]
Qdenga (formerly TAK-003) completed clinical trials in 2022 and was approved for use in the European Union in December 2022;[53] it has been approved by a number of other countries including Indonesia and Brazil, and has been recommended by the SAGE committee of the World Health Organization.[58] It is indicated for the prevention of dengue disease in individuals four years of age and older, and can be administered to people who have not been previously infected with dengue. It is a live attenuated vaccine containing the four serotypes of dengue virus, administered subcutaneously as two doses three months apart.[53]
Severe disease
[edit]The World Health Organization's International Classification of Diseases divides dengue fever into two classes: uncomplicated and severe.[18] Severe dengue is defined as that associated with severe bleeding, severe organ dysfunction, or severe plasma leakage.[59]
Severe dengue can develop suddenly, sometimes after a few days as the fever subsides.[24] Leakage of plasma from the capillaries results in extreme low blood pressure and hypovolemic shock; Patients with severe plasma leakage may have fluid accumulation in the lungs or abdomen, insufficient protein in the blood, or thickening of the blood. Severe dengue is a medical emergency which can cause damage to organs, leading to multiple organ failure and death.[60]
Diagnosis
[edit]Mild cases of dengue fever can easily be confused with several common diseases including Influenza, measles, chikungunya, and zika.[61][62] Dengue, chikungunya and zika share the same mode of transmission (Aedes mosquitoes) and are often endemic in the same regions, so that it is possible to be infected simultaneously by more than one disease.[63] For travellers, dengue fever diagnosis should be considered in anyone who develops a fever within two weeks of being in the tropics or subtropics.[21]

Warning symptoms of severe dengue include abdominal pain, persistent vomiting, odema, bleeding, lethargy, and liver enlargement. Once again, these symptoms can be confused with other diseases such as malaria, gastroenteritis, leptospirosis, and typhus.[61]
Blood tests can be used to confirm a diagnosis of dengue. During the first few days of infection, enzyme-linked immunosorbent assay (ELISA) can be used to detect the NS1 antigen; however this antigen is produced by all flaviviruses.[63][11] Four or five days into the infection, it is possible to reliably detect anti-dengue IgM antibodies, but this does not determine the serotype.[63] Nucleic acid amplification tests provide the most reliable method of diagnosis.[11]
Treatment
[edit]As of July 2024, there is no specific antiviral treatment available for dengue fever.[64]
Most cases of dengue fever have mild symptoms, and recovery takes place in a few days.[1] No treatment is required for these cases. Acetaminophen (Paracetamol, Tylenol) may be used to relieve mild fever or pain. Other common pain relievers, including aspirin, ibuprofen (Advil, Motrin IB, others) and naproxen sodium (Aleve) should be avoided as they can increase the risk of bleeding complications.[64]
For moderate illness, those who can drink, are passing urine, have no warning signs and are otherwise reasonably healthy can be monitored carefully at home. Supportive care with analgesics, fluid replacement, and bed rest are recommended.[65][23]
Severe dengue is a life-threatening emergency, requiring hospitalization and potentially intensive care.[24] Warning signs include dehydration, decreasing platelets and increasing hematocrit.[66] Treatment modes include intravenous fluids, and transfusion with platelets or plasma.[65]
Prognosis
[edit]Most people with dengue recover without any ongoing problems. The risk of death among those with severe dengue is 0.8–2.5%,[67] and with adequate treatment this is less than 1%. However, those who develop significantly low blood pressure may have a fatality rate of up to 26%.[23] The risk of death among children less than five years old is four times greater than among those over the age of 10.[67] Elderly people are also at higher risk of a poor outcome.[67]
Epidemiology
[edit]
As of March 2023, dengue is endemic in more than 100 countries with cases reported in every continent with the exception of Antarctica.[1][68] The Americas, Southeast Asia and the Western Pacific regions are the most seriously affected.[1][69] It is difficult to estimate the full extent of the disease, as many cases are mild and not correctly diagnosed. WHO currently estimates that 3.9 billion people are at risk of dengue infection.[1][69] In 2013, it was estimated that 390 million dengue infections occur every year, with 500,000 of these developing severe symptoms and 25,000 deaths.[70][71]
| External image | |
|---|---|
| An online map showing reports of dengue fever | |
Generally, areas where dengue is endemic have only one serotype of the virus in circulation. The disease is said to be hyperendemic in areas where more than one serotype is circulating; this increases the risk of severe disease on a second or subsequent infection.[72]
Infections are most commonly acquired in urban environments where the virus is primarily transmitted by the mosquito species Aedes aegypti.[73] This species has adapted to the urban environment, is generally found close to human habitation, prefers humans as its host, and takes advantage of small bodies of standing water (such as tanks and buckets) in which to breed. In rural settings the virus is transmitted to humans by A. aegypti and other related mosquitoes such as Aedes albopictus.[73] Both these species have expanding ranges.[21] There are two subspecies of Aedes aegypti, where Aedes aegypti formosus can be found in natural habitats such as forests and Aedes Aegypti aegypti has adapted to urban domestic habitats.[74]
Dengue has increased in incidence in recent decades, with WHO recording a ten fold increase between 2010 and 2019 (from 500,000 to 5 million recorded cases).[1] This increase is tied closely to the increasing range of Aedes mosquitoes, which is attributed to a combination of urbanization, population growth, and an increasingly warm climate.[75][76] In endemic areas, dengue infections peak when rainfall is optimal for mosquito breeding.[77] In October 2023, the first confirmed symptomatic case of locally acquired dengue (i.e. not while travelling) in the US was identified in California.[78]
The disease infects all races, sexes, and ages equally. In endemic areas, the infection is most commonly seen in children who then acquire a lifelong partial immunity.[71]
History
[edit]The first historical record of a case of probable dengue fever is in a Chinese medical encyclopedia from the Jin dynasty (266–420) which referred to a "water poison" associated with flying insects.[79][80]
The principal mosquito vector of dengue, Aedes aegypti, spread out of Africa in the 15th to 19th centuries due to the slave trade and consequent expansion of international trading.[21] There have been descriptions of epidemics of dengue-like illness in the 17th century, and it is likely that epidemics in Jakarta, Cairo, and Philadelphia during the 18th century were caused by dengue.[79][81]
It is assumed that dengue was constantly present in many tropical urban centres throughout the 19th and early 20th centuries, even though significant outbreaks were infrequent.[79] The marked spread of dengue during and after the Second World War has been attributed partly to disruption caused by the war, and partly to subsequent urbanisation in south-east Asia.[79] As novel serotypes were introduced to regions already endemic with dengue, outbreaks of severe disease followed. The severe hemorrhagic form of the disease was first reported in the Philippines in 1953; by the 1970s, it had become recognised as a major cause of child mortality in Southeast Asia.[79]
In Central and South America, the Aedes mosquito had been eradicated in the 1950s; however the eradication program was discontinued in the 1970s and the disease re-established itself in the region during the 1980s, becoming hyperendemic and causing significant epidemics.[79]
Dengue has continued to increase in prevalence during the 21st century, as the mosquito vector continues to expand its range. This is attributed partly to continuing urbanisation, and partly to the impact of a warmer climate.[82]
Etymology
[edit]The name came into English in the early 19th century from West Indian Spanish, which borrowed it from the Kiswahili term dinga / denga, meaning "cramp-like seizure" – the full term of the condition being ki-dinga pepo: "a sort of cramp-like seizure (caused by) an evil spirit".[83] The borrowed term changed to dengue in Spanish due to this word existing in Spanish with the meaning "fastidiousness" and this folk etymology referring to the dislike of movement by affected patients.[4][84] Slaves in the West Indies having contracted dengue were said to have the posture and gait of a dandy, and the disease was known as "dandy fever".[85][86]
The term break-bone fever was applied by physician and United States Founding Father Benjamin Rush, in a 1789 report of the 1780 epidemic in Philadelphia, due to the associated muscle and joint pains. In the report title he uses the more formal term "bilious remitting fever".[87] The term dengue fever came into general use only after 1828.[86] Other historical terms include "breakheart fever" and "la dengue".[86] Terms for severe disease include "infectious thrombocytopenic purpura" and "Philippine", "Thai", or "Singapore hemorrhagic fever".[86]
Research
[edit]Research directions include dengue pathogenesis (the process by which the disease develops in humans), as well as the biology, ecology and behaviour of the mosquito vector. Improved diagnostics would enable faster and more appropriate treatment.[88] Attempts are ongoing to develop an antiviral medicine targeting the NS3 or NS5 proteins.[89]
In addition to the two vaccines which are already available, several vaccine candidates are in development.[90]
Society and culture
[edit]Blood donation
[edit]Outbreaks of dengue fever increase the need for blood products while decreasing the number of potential blood donors due to potential infection with the virus.[91] Someone who has a dengue infection is typically not allowed to donate blood for at least the next six months.[91]
Public awareness
[edit]
International Anti-Dengue Day is observed every year on 15 June in a number of countries.[92] The idea was first agreed upon in 2010 with the first event held in Jakarta, Indonesia, in 2011.[92] Further events were held in 2012 in Yangon, Myanmar, and in 2013 in Vietnam.[92] Goals are to increase public awareness about dengue, mobilize resources for its prevention and control and, to demonstrate the Southeast Asian region's commitment in tackling the disease.[93] Efforts are ongoing as of 2019 to make it a global event.[94]
The Philippines has an awareness month in June since 1998.[95][96]
A National Dengue Day is held in India annually on 16 May.[97]
Economic burden
[edit]A study estimated that the global burden of dengue in 2013 amounted to US$8.9 billion.[98]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m n o p q r s "Dengue and severe dengue". World Health Organization. 17 March 2023. Archived from the original on 14 March 2024. Retrieved 10 February 2024.
- ^ a b c d e f g h Kularatne SA (September 2015). "Dengue fever". BMJ. 351: h4661. doi:10.1136/bmj.h4661. PMID 26374064. S2CID 1680504.
- ^ dengue Archived 17 July 2022 at the Wayback Machine in the Merriam-Webster Dictionary
- ^ a b dengue in Oxford Dictionaries
- ^ Nelson Textbook of Pediatrics: The field of pediatrics. Elsevier Health Sciences. 2016. p. 1631. ISBN 978-1-4557-7566-8. Archived from the original on 10 September 2017.
- ^ East S (6 April 2016). "World's first dengue fever vaccine launched in the Philippines". CNN. Archived from the original on 18 October 2016. Retrieved 17 October 2016.
- ^ a b c d e "Dengue- Global situation". World Health Organization. Archived from the original on 13 February 2024. Retrieved 13 February 2024.
- ^ Alejandria MM (April 2015). "Dengue haemorrhagic fever or dengue shock syndrome in children". BMJ Clinical Evidence. 2015: 0917. PMC 4392842. PMID 25860404.
- ^ a b "Dengue | CDC Yellow Book 2024". Centers for Disease Control and Prevention. 1 May 2023. Archived from the original on 14 February 2024. Retrieved 14 February 2024.
- ^ Schaefer TJ, Panda PK, Wolford RW (14 November 2022). "Dengue Fever". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 28613483. Archived from the original on 14 December 2021. Retrieved 13 February 2022.
- ^ a b c "Dengue Diagnosis | CDC". Centers for Disease Control and Prevention. 13 June 2019. Archived from the original on 14 February 2024. Retrieved 14 February 2024.
- ^ a b "Dengue: How to keep children safe | UNICEF South Asia". www.unicef.org. Archived from the original on 13 February 2024. Retrieved 13 February 2024.
- ^ "Dengue vaccine: WHO position paper – September 2018" (PDF). Weekly Epidemiological Record. 36 (93): 457–76. 7 September 2018. Archived (PDF) from the original on 5 January 2019. Retrieved 12 April 2019.
- ^ "Qdenga | European Medicines Agency". European Medicines Agency. 16 December 2022. Archived from the original on 27 February 2024. Retrieved 14 February 2024.
- ^ Henchal EA, Putnak JR (October 1990). "The dengue viruses". Clinical Microbiology Reviews. 3 (4): 376–396. doi:10.1128/CMR.3.4.376. PMC 358169. PMID 2224837.
- ^ Paz-Bailey G, Adams LE, Deen J, Anderson KB, Katzelnick LC (February 2024). "Dengue". Lancet. 403 (10427): 667–682. doi:10.1016/S0140-6736(23)02576-X. PMID 38280388. S2CID 267201333.
- ^ "Neglected tropical diseases". World Health Organization. Archived from the original on 26 January 2024. Retrieved 13 February 2024.
- ^ a b c Whitehorn J, Farrar J (2010). "Dengue". British Medical Bulletin. 95: 161–173. doi:10.1093/bmb/ldq019. PMID 20616106. S2CID 215154729.
- ^ a b Reiter P (March 2010). "Yellow fever and dengue: a threat to Europe?". Euro Surveillance. 15 (10): 19509. doi:10.2807/ese.15.10.19509-en. PMID 20403310. Archived from the original on 7 July 2011.
- ^ a b Gubler DJ (2010). "Dengue viruses". In Mahy BW, Van Regenmortel MH (eds.). Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. pp. 372–82. ISBN 978-0-12-375147-8. Archived from the original on 9 June 2016.
- ^ a b c d e f g h Simmons CP, Farrar JJ, Nguyen V, Wills B (April 2012). "Dengue" (PDF). The New England Journal of Medicine. 366 (15): 1423–1432. doi:10.1056/NEJMra1110265. hdl:11343/191104. PMID 22494122. Archived from the original on 28 August 2021. Retrieved 24 September 2019.
- ^ a b Chen LH, Wilson ME (October 2010). "Dengue and chikungunya infections in travelers". Current Opinion in Infectious Diseases. 23 (5): 438–444. doi:10.1097/QCO.0b013e32833c1d16. PMID 20581669. S2CID 2452280.
- ^ a b c d e f Ranjit S, Kissoon N (January 2011). "Dengue hemorrhagic fever and shock syndromes". Pediatric Critical Care Medicine. 12 (1): 90–100. doi:10.1097/PCC.0b013e3181e911a7. PMID 20639791. S2CID 10135251.
- ^ a b c d e "Dengue fever - Symptoms and causes". Mayo Clinic. Archived from the original on 26 February 2024. Retrieved 25 February 2024.
- ^ Kalimuddin S, Teh YE, Wee LE, Paintal S, Sasisekharan R, Low JG, et al. (August 2022). "Chronic sequelae complicate convalescence from both dengue and acute viral respiratory illness". PLOS Neglected Tropical Diseases. 16 (8): e0010724. doi:10.1371/journal.pntd.0010724. PMC 9426910. PMID 35981059.
- ^ Paixão ES, Teixeira MG, Costa MD, Rodrigues LC (July 2016). "Dengue during pregnancy and adverse fetal outcomes: a systematic review and meta-analysis". The Lancet. Infectious Diseases. 16 (7): 857–865. doi:10.1016/S1473-3099(16)00088-8. PMID 26949028. Archived from the original on 28 August 2021. Retrieved 5 December 2019.
- ^ Rothman AL (July 2011). "Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms". Nature Reviews. Immunology. 11 (8): 532–543. doi:10.1038/nri3014. PMID 21760609.
- ^ Zanluca, C., & Duarte dos Santos, C. N. (2016). Zika virus – an overview. Microbes and Infection, 18(5), 295-301. Retrieved 27 September 2024
- ^ Rodenhuis-Zybert IA, Wilschut J, Smit JM (August 2010). "Dengue virus life cycle: viral and host factors modulating infectivity" (PDF). Cellular and Molecular Life Sciences. 67 (16): 2773–2786. doi:10.1007/s00018-010-0357-z. PMC 11115823. PMID 20372965. S2CID 4232236. Archived (PDF) from the original on 9 October 2022.
- ^ a b Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. (December 2010). "Dengue: a continuing global threat". Nature Reviews. Microbiology. 8 (12 Suppl): S7-16. doi:10.1038/nrmicro2460. PMC 4333201. PMID 21079655.
- ^ Solomonides T (2010). Healthgrid applications and core technologies : proceedings of HealthGrid 2010 ([Online-Ausg.] ed.). Amsterdam: IOS Press. p. 235. ISBN 978-1-60750-582-2. Archived from the original on 1 May 2016.
- ^ "Aedes aegypti – Factsheet for experts". www.ecdc.europa.eu. 9 June 2017. Archived from the original on 10 July 2017. Retrieved 25 February 2024.
- ^ Global Strategy For Dengue Prevention And Control (PDF). World Health Organization. 2012. pp. 16–17. ISBN 978-92-4-150403-4. Archived (PDF) from the original on 30 October 2012.
- ^ "Travelers' Health Outbreak Notice". Centers for Disease Control and Prevention. 2 June 2010. Archived from the original on 26 August 2010. Retrieved 27 August 2010.
- ^ Gould EA, Solomon T (February 2008). "Pathogenic flaviviruses". Lancet. 371 (9611): 500–509. doi:10.1016/S0140-6736(08)60238-X. PMID 18262042. S2CID 205949828. Archived from the original on 28 August 2021. Retrieved 6 June 2020.
- ^ Gwee SX, St John AL, Gray GC, Pang J (June 2021). "Animals as potential reservoirs for dengue transmission: A systematic review". One Health. 12: 100216. doi:10.1016/j.onehlt.2021.100216. PMC 7868715. PMID 33598525.
- ^ "Vector-borne viral infections". World Health Organization. Archived from the original on 3 February 2011. Retrieved 17 January 2011.
- ^ a b Center for Disease Control and Prevention. "Chapter 5 – dengue fever (DF) and dengue hemorrhagic fever (DHF)". 2010 Yellow Book. Archived from the original on 29 December 2010. Retrieved 23 December 2010.
- ^ St Georgiev V (2009). National Institute of Allergy and Infectious Diseases, NIH (1 ed.). Totowa, N.J.: Humana. p. 268. ISBN 978-1-60327-297-1. Archived from the original on 1 May 2016.
- ^ "Dengue Transmission | Learn Science at Scitable". © 2014 Nature Education. Archived from the original on 28 February 2024. Retrieved 28 February 2024.
- ^ Wiwanitkit V (November 2009). "Unusual mode of transmission of dengue". Journal of Infection in Developing Countries. 4 (1): 51–54. doi:10.3855/jidc.145. PMID 20130380.
- ^ a b c Bisen PS, Raghuvanshi R (22 July 2013). Emerging Epidemics: Management and Control (1 ed.). Wiley. doi:10.1002/9781118393277.ch8. ISBN 978-1-118-39323-9. Archived from the original on 23 February 2024. Retrieved 23 February 2024.
- ^ "Host Response to the Dengue Virus | Learn Science at Scitable". www.nature.com. Archived from the original on 23 February 2024. Retrieved 23 February 2024.
- ^ a b Teo A, Tan HD, Loy T, Chia PY, Chua CL (March 2023). "Understanding antibody-dependent enhancement in dengue: Are afucosylated IgG1s a concern?". PLOS Pathogens. 19 (3): e1011223. doi:10.1371/journal.ppat.1011223. PMC 10062565. PMID 36996026.
- ^ Teo A, Tan HD, Loy T, Chia PY, Chua CL (March 2023). "Understanding antibody-dependent enhancement in dengue: Are afucosylated IgG1s a concern?". PLOS Pathogens. 19 (3): e1011223. doi:10.1371/journal.ppat.1011223. PMC 10062565. PMID 36996026.
- ^ "Antibody-dependent Enhancement (ADE) and Vaccines". The Children's Hospital of Philadelphia. 7 December 2020. Archived from the original on 29 February 2024. Retrieved 29 February 2024.
- ^ "Host Response to the Dengue Virus | Learn Science at Scitable". © 2014 Nature Education. Archived from the original on 23 February 2024. Retrieved 28 February 2024.
- ^ a b Martina BE, Koraka P, Osterhaus AD (October 2009). "Dengue virus pathogenesis: an integrated view". Clinical Microbiology Reviews. 22 (4): 564–581. doi:10.1128/CMR.00035-09. PMC 2772360. PMID 19822889.
- ^ Center for Disease Control and Prevention. "Chapter 5 – dengue fever (DF) and dengue hemorrhagic fever (DHF)". 2010 Yellow Book. Archived from the original on 29 December 2010. Retrieved 23 December 2010.
- ^ a b CDC (20 October 2023). "Traveling? Avoid Dengue". Centers for Disease Control and Prevention. Archived from the original on 8 March 2024. Retrieved 8 March 2024.
- ^ Reiter P (March 2010). "Yellow fever and dengue: a threat to Europe?". Euro Surveillance. 15 (10): 19509. doi:10.2807/ese.15.10.19509-en. PMID 20403310. Archived from the original on 7 July 2011.
- ^ a b "Controlling Dengue Outbreaks | Learn Science at Scitable". www.nature.com. Archived from the original on 8 March 2024. Retrieved 8 March 2024.
- ^ a b c "Factsheet about dengue". www.ecdc.europa.eu. 7 August 2023. Archived from the original on 25 February 2024. Retrieved 8 March 2024.
- ^ "Information on Dengvaxia®". www.sanofi.com. Archived from the original on 8 March 2024. Retrieved 8 March 2024.
- ^ "Dengvaxia : EPAR - Medicine overview" (PDF). European Medicines Agency. Archived (PDF) from the original on 9 March 2024. Retrieved 9 March 2024.
- ^ "Sanofi restricts dengue vaccine but downplays antibody enhancement | CIDRAP". Center for Infectious Disease Research & Policy. 1 December 2017. Archived from the original on 9 March 2024. Retrieved 9 March 2024.
- ^ "Administering the Dengue Vaccine". www.cdc.gov. 26 April 2023. Archived from the original on 8 March 2024. Retrieved 8 March 2024.
- ^ "Message by the Director of the Department of Immunization, Vaccines and Biologicals at WHO - September 2023". World Health Organization. Archived from the original on 16 March 2024. Retrieved 8 March 2024.
- ^ "International Classification of Diseases (ICD-11)". World Health Organization. 1 January 2022. Archived from the original on 20 September 2023. Retrieved 28 February 2024.
- ^ CDC (13 April 2023). "Dengue Clinical Presentation | CDC". Centers for Disease Control and Prevention. Archived from the original on 29 February 2024. Retrieved 29 February 2024.
- ^ a b Organization WH (21 April 2009). Dengue guidelines, for diagnosis, treatment, prevention and control. World Health Organization. p. 46. ISBN 978-92-4-154787-1. Archived from the original on 16 March 2024. Retrieved 7 March 2024.
Textbox B and Textbox C.
- ^ "Dengue - Infectious Diseases". MSD Manual Professional Edition. Archived from the original on 4 March 2024. Retrieved 4 March 2024.
- ^ a b c Beltrán-Silva SL, Chacón-Hernández SS, Moreno-Palacios E, Pereyra-Molina JÁ (1 July 2018). "Clinical and differential diagnosis: Dengue, chikungunya and Zika". Revista Médica del Hospital General de México. 81 (3): 146–153. doi:10.1016/j.hgmx.2016.09.011. ISSN 0185-1063. Archived from the original on 4 March 2024. Retrieved 7 March 2024.
- ^ a b "Dengue fever - Diagnosis and treatment". Mayo Clinic. 5 October 2022. Archived from the original on 10 March 2024. Retrieved 10 March 2024.
- ^ a b Smith DS, Mariano DJ, Trautwein ML (16 November 2022). Bronze MS (ed.). "Dengue Treatment & Management". Medscape. Archived from the original on 10 March 2024. Retrieved 10 March 2024.
- ^ "Dengue Case Management" (PDF). Centers for Disease Control and Prevention. Archived (PDF) from the original on 10 March 2024. Retrieved 10 March 2024.
- ^ a b c Kularatne SA (September 2015). "Dengue fever". BMJ. 351: h4661. doi:10.1136/bmj.h4661. PMID 26374064. S2CID 1680504.
- ^ CDC (21 September 2023). "Dengue Areas of Risk Around the World | CDC". Centers for Disease Control and Prevention. Retrieved 17 March 2024.
- ^ a b Tidy C (22 June 2022). Vakharia K (ed.). "Dengue (Causes, Symptoms, and Treatment)". patient.info. Retrieved 14 March 2024.
- ^ Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. (April 2013). "The global distribution and burden of dengue". Nature. 496 (7446): 504–507. Bibcode:2013Natur.496..504B. doi:10.1038/nature12060. PMC 3651993. PMID 23563266.
- ^ a b Jing Q, Wang M (June 2019). "Dengue epidemiology". Global Health Journal. 3 (2): 37–45. doi:10.1016/j.glohj.2019.06.002. ISSN 2414-6447.
- ^ Smith DS. "What is characteristic of hyperendemic dengue?". Medscape. Retrieved 23 June 2021.
- ^ a b Gubler DJ (2010). "Dengue viruses". In Mahy BW, Van Regenmortel MH (eds.). Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. pp. 372–82. ISBN 978-0-12-375147-8. Archived from the original on 9 June 2016.
- ^ "Aedes aegypti - Factsheet for experts". www.ecdc.europa.eu. 9 June 2017. Retrieved 18 October 2024.
- ^ Whitehorn J, Farrar J (2010). "Dengue". British Medical Bulletin. 95: 161–173. doi:10.1093/bmb/ldq019. PMID 20616106. S2CID 215154729.
- ^ "Climate Change Will Expose Half of World's Population to Disease-Spreading Mosquitoes By 2050". Yale Environment 360. 5 March 2019. Retrieved 17 March 2024.
- ^ "Dengue increase likely during rainy season: WHO warns". World Health Organization. Retrieved 17 March 2024.
- ^ Feaster M, Patrick R, Oshiro M, Kuan M, Goh YY, Carmona M, et al. (October 2024). "Notes from the Field: First Locally Acquired Dengue Virus Infections - Pasadena, California, October-December 2023". MMWR. Morbidity and Mortality Weekly Report. 73 (42): 955–956. doi:10.15585/mmw.mm7342a4. PMC 11500840. PMID 39446691.
- ^ a b c d e f Gubler DJ (July 1998). "Dengue and dengue hemorrhagic fever". Clinical Microbiology Reviews. 11 (3): 480–496. doi:10.1128/cmr.11.3.480. PMC 88892. PMID 9665979.
- ^ Anonymous (2006). "Etymologia: dengue" (PDF). Emerg. Infect. Dis. 12 (6): 893. doi:10.3201/eid1206.ET1206. PMC 3373045. S2CID 29398958. Archived (PDF) from the original on 3 December 2013.
- ^ Kuno G (November 2015). "A Re-Examination of the History of Etiologic Confusion between Dengue and Chikungunya". PLOS Neglected Tropical Diseases. 9 (11): e0004101. doi:10.1371/journal.pntd.0004101. PMC 4643049. PMID 26562299.
1779–1780: 'Knokkel-koorts' in Batavia (now Jakarta, Indonesia) and 'break bone fever' in Philadelphia
- ^ "As Temperatures Rise, Dengue Fever Spreads and Cases Rise". Yale E360. Retrieved 20 March 2024.
- ^ Christie J. On Epidemics of Dengue Fever: Their Diffusion and Etiology. Glasgow Med J. 1881;16(3):161-176.
- ^ Anonymous (2006). "Etymologia: dengue" (PDF). Emerg. Infect. Dis. 12 (6): 893. doi:10.3201/eid1206.ET1206. PMC 3373045. S2CID 29398958. Archived (PDF) from the original on 3 December 2013.
- ^ Anonymous (15 June 1998). "Definition of Dandy fever". MedicineNet.com. Archived from the original on 5 June 2011. Retrieved 25 December 2010.
- ^ a b c d Halstead SB (2008). Dengue (Tropical Medicine: Science and Practice). River Edge, N.J: Imperial College Press. pp. 1–10. ISBN 978-1-84816-228-0. Archived from the original on 21 May 2016.
- ^ Barrett AD, Stanberry LR (2009). Vaccines for biodefense and emerging and neglected diseases. San Diego: Academic. pp. 287–323. ISBN 978-0-12-369408-9. Archived from the original on 12 May 2016.
- ^ Laughlin CA, Morens DM, Cassetti MC, Costero-Saint Denis A, San Martin JL, Whitehead SS, et al. (October 2012). "Dengue research opportunities in the Americas". The Journal of Infectious Diseases. 206 (7): 1121–1127. doi:10.1093/infdis/jis351. PMC 3499110. PMID 22782946.
- ^ Obi JO, Gutiérrez-Barbosa H, Chua JV, Deredge DJ (September 2021). "Current Trends and Limitations in Dengue Antiviral Research". Tropical Medicine and Infectious Disease. 6 (4): 180. doi:10.3390/tropicalmed6040180. PMC 8544673. PMID 34698303.
- ^ Kariyawasam R, Lachman M, Mansuri S, Chakrabarti S, Boggild AK (20 April 2023). "A dengue vaccine whirlwind update". Therapeutic Advances in Infectious Disease. 10: 20499361231167274. doi:10.1177/20499361231167274. PMC 10126642. PMID 37114191.
- ^ a b Teo D, Ng LC, Lam S (April 2009). "Is dengue a threat to the blood supply?". Transfusion Medicine. 19 (2): 66–77. doi:10.1111/j.1365-3148.2009.00916.x. PMC 2713854. PMID 19392949.
- ^ a b c "Marking ASEAN Dengue Day". Archived from the original on 17 June 2015. Retrieved 16 June 2015.
- ^ ACTION AGAINST DENGUE Dengue Day Campaigns Across Asia. World Health Organization. 2011. ISBN 978-92-9061-539-2.
- ^ "Calling for a World Dengue Day". The International Society for Neglected Tropical Diseases. Archived from the original on 7 January 2019. Retrieved 7 January 2019.
- ^ "Dengue Awareness Month | GOVPH". Official Gazette of the Republic of the Philippines. Official Gazette. 2 June 2011. Archived from the original on 7 January 2019. Retrieved 7 January 2019.
- ^ "Did you know: Dengue Awareness Month | Inquirer News". newsinfo.inquirer.net. 9 June 2015. Archived from the original on 7 January 2019. Retrieved 7 January 2019.
- ^ "National Dengue Day". 6 June 2018. Archived from the original on 7 January 2019. Retrieved 7 January 2019.
- ^ Shepard DS, Undurraga EA, Halasa YA, Stanaway JD (August 2016). "The global economic burden of dengue: a systematic analysis". The Lancet. Infectious Diseases. 16 (8): 935–941. doi:10.1016/s1473-3099(16)00146-8. PMID 27091092.




