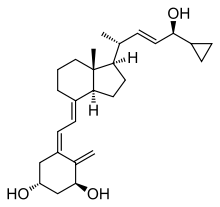
Calcipotriol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Daivonex, Dovonex, Sorilux |
| Other names | calcipotriene (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608018 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Topical administration |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 5 to 6% |
| Metabolism | Liver |
| Excretion | Biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.119.473 |
| Chemical and physical data | |
| Formula | C27H40O3 |
| Molar mass | 412.614 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Calcipotriol, also known as calcipotriene, is a synthetic derivative of calcitriol, a form of vitamin D. It is used in the treatment of psoriasis.[1] It is safe for long-term application in psoriatic skin conditions.[medical citation needed]
It was patented in 1985 and approved for medical use in 1991.[2] It is marketed under the trade name "Dovonex" in the United States, "Daivonex" outside North America, and "Psorcutan" in Germany.[citation needed]
It is on the World Health Organization's List of Essential Medicines.[3]
Calcipotriol is also available as Calcipotriol/betamethasone dipropionate, a fixed-dose combination medication with the synthetic corticosteroid betamethasone dipropionate for the treatment of plaque psoriasis.[4]
Medical uses
[edit]Chronic plaque psoriasis is the chief medical use of calcipotriol.[5] It has also been used successfully in the treatment of alopecia areata.[6]
Contraindications
[edit]Hypersensitivity, use on face, hypercalcaemia, or evidence of vitamin D toxicity are the only contraindications for calcipotriol use.[7]
Cautions include exposure to excessive natural or artificial light, due to the potential for calcipotriol to cause photosensitivity.[7]
Adverse effects
[edit]Adverse effects by frequency:[5][7][8][9]
- Very common (> 10% frequency)
- Burning
- Itchiness
- Skin irritation
- Common (1–10% frequency)
- Dermatitis
- Dry skin
- Erythema
- Peeling
- Worsening of psoriasis including facial/scalp
- Rash
- Uncommon (0.1–1% frequency)
- Exacerbation of psoriasis
- Rare (< 0.1% frequency)
- Allergic contact dermatitis
- Hypercalcaemia
- Photosensitivity
- Changes in pigmentation
- Skin atrophy
Interactions
[edit]No drug interactions are known.[7]
Pharmacology
[edit]Mechanism of action
[edit]The efficacy of calcipotriol in the treatment of psoriasis was first noticed by the observation of patients receiving various forms of vitamin D in an osteoporosis study. Unexpectedly, some patients who also had psoriasis experienced dramatic reductions in lesion counts.[10]
The precise mechanism of calcipotriol in remitting psoriasis is not well understood. However, it has been shown to have comparable affinity with calcitriol for the vitamin D receptor (VDR), while being less than 1% as active as the calcitriol in regulating calcium metabolism. The vitamin D receptor belongs to the steroid/thyroid receptor superfamily, and is found on the cells of many different tissues including the thyroid, bone, kidney, and T cells of the immune system. T cells are known to play a role in psoriasis, and it is thought that the binding of calcipotriol to the VDR modulates the T cells gene transcription of cell differentiation and proliferation related genes.
In mouse studies, topical calcipotriol administration to the ear and dorsal skin led to a dose-dependent increase in the production of the epithelial cell-derived cytokine TSLP by keratinocytes, and triggered atopic dermatitis at high concentrations.[11] This upregulation of TSLP production due to calcipotriol application is thought to be mediated through the coactivation of vitamin D receptor/RXRα and vitamin D receptor/RXRβ heterodimers. As psoriasis is typically thought to be partially driven by Th1/Th17 inflammatory cytokines,[12] calcipotriol treatment at appropriate concentrations may alleviate psoriasis symptoms by repressing Th1/Th17 inflammation through TSLP production, which is linked to a Th2 response. However, it is important to note that this has not yet been confirmed.
Pharmacokinetics
[edit]After application and systemic uptake, calcipotriol undergoes rapid hepatic metabolism. Calcipotriol is metabolized to MC1046 (the α,β−unsaturated ketone analog), which is subsequently metabolized to its primary metabolite, the saturated ketone analog MC1080. MC1080 is then slowly metabolized to calcitroic acid.[13]
The metabolites of calcipotriol are less potent than the parent compound.
References
[edit]- ^ Prendergast B, Harrison J, Kulkarni K, Baguneid M (2016-10-27). Oxford Handbook of Key Clinical Evidence. Oxford University Press. p. 101. ISBN 9780198729426.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 452. ISBN 9783527607495.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "Taclonex- calcipotriene and betamethasone dipropionate ointment". DailyMed. 21 May 2020. Retrieved 19 October 2020.
- ^ a b Rossi S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ^ Kim DH, Lee JW, Kim IS, Choi SY, Lim YY, Kim HM, et al. (August 2012). "Successful treatment of alopecia areata with topical calcipotriol". Annals of Dermatology. 24 (3): 341–344. doi:10.5021/ad.2012.24.3.341. PMC 3412244. PMID 22879719.
- ^ a b c d "Dovonex, Calcitrene Ointment (calcipotriene) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 26 January 2014.
- ^ "CALCIPOTRIENE (calcipotriene) solution [E. FOUGERA & CO. A division of Fougera Pharmaceuticals Inc.]". DailyMed. E. FOUGERA & CO. A division of Fougera Pharmaceuticals Inc. May 2012. Retrieved 26 January 2014.
- ^ "PRODUCT INFORMATION DAIVONEX® CREAM Calcipotriol 50 microgram/g" (PDF). TGA eBusiness Services. LEO Pharma Pty Ltd. 28 April 2011. Retrieved 26 January 2014.
- ^ Morimoto S, Kumahara Y (March 1985). "A patient with psoriasis cured by 1 alpha-hydroxyvitamin D3". Medical Journal of Osaka University. 35 (3–4): 51–54. PMID 4069059.
- ^ Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P (August 2006). "Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis". Proceedings of the National Academy of Sciences of the United States of America. 103 (31): 11736–11741. Bibcode:2006PNAS..10311736L. doi:10.1073/pnas.0604575103. PMC 1544239. PMID 16880407.
- ^ Wong T, Hsu L, Liao W (2013-02-01). "Phototherapy in psoriasis: a review of mechanisms of action". Journal of Cutaneous Medicine and Surgery. 17 (1): 6–12. doi:10.2310/7750.2012.11124. PMC 3736829. PMID 23364144.
- ^ "Enstilar (calcipotriene and betamethasone dipropionate) Foam, 0.005%/0.064% for topical use. Full Prescribing Information" (PDF). Parsippany, NJ: LEO Pharma Inc. 2015. Archived from the original (PDF) on 2018-09-20. Retrieved 2015-11-21.
External links
[edit]- "Calcipotriene". Drug Information Portal. U.S. National Library of Medicine.
