Cicletanine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 99.6% for (-)cicletanine isomer; 87.5% for (+)cicletanine isomer |
| Elimination half-life | 5.7 h. Also: Tmax = 0.750 hours. Plasma AUCinf = 29.0 μg·hr/mL. Cmax = 6.18 μg/mL. All figures are for a single oral dose of 150 mg. |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.583 |
| Chemical and physical data | |
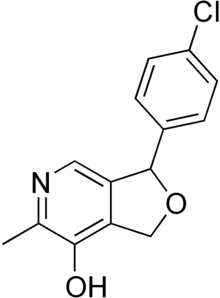
| Formula | C14H12ClNO2 |
| Molar mass | 261.71 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cicletanine is a furopyridine compound approved in France for the treatment of hypertension.[1] The drug is most commonly known as a diuretic drug, but has a broader range of cardiovascular and metabolic activity characterized extensively in the literature (see "Mechanism" below).
Cicletanine was originated and in 1986 launched in France by Paris-based Ipsen, who in 2005 licensed marketing rights in France to Milan-based Recordati for several years (at least until 2010). Ipsen and Recordati both marketed cicletanine under the trade name Tenstaten. The drug is no longer manufactured nor sold by IPSEN; it is currently marketed in France by three generics manufacturers: Viatris, Biogaran, and Teva Pharmaceuticals.
Cicletanine has been shown to be differentially effective in salt-sensitive hypertension.[2]
Mechanism
[edit]According to its current French package insert, cicletanine is officially in the category of “Other cortical segment diuretics.” (French: “Autres diurétiques du segment cortical de dilution.”) Internationally, cicletanine’s ATC Code C03BX03 is a subcategory under C03BX “Other low-ceiling diuretics.” This categorization as a diuretic (without mention of other activity) is despite (1) very early indications that the drug had therapeutic activity beyond diuresis (in some cases at sub-diuretic doses) and (2) subsequent findings on multiple mechanisms of vasorelaxation. While cicletanine's first peer-reviewed publication (Lancet, 1983) reported diuretic activity, it associated that activity with prostacyclin, at the time known more as an important vasodilator than as a diuretic agent. In fact, the Nobel Prize in Physiology or Medicine was given the prior year to Sune Bergström, Bengt Samuelsson and John Vane for work on prostaglandins, including prostacyclin, which Vane had demonstrated to be a vasodilator and inhibitor of platelet aggregation.
Cicletanine's official categorization as a diuretic notwithstanding, the drug's antihypertensive activity as experienced in most patients involves relaxation of arteries more than decrease of vascular-fluid volume (i.e., via diuresis). A significant majority of cicletanine patients take daily doses of 50 or 100 mg, while 150 mg is considered the minimum dose for diuretic activity. One market analysis reported that 66% of cicletanine patients were taking 50 mg / day and 33% were taking 100 mg / day. Consistent with clinical efficacy at sub-diuretic levels, cicletanine doses of 50 or 100 mg have been successful in significantly decreasing blood pressure in clinical trials involving (in aggregate) thousands of patients.
While a comprehensive survey of cicletanine's mechanism of action has yet to be published, it is now known that the drug's vasorelaxant activity is due to (1) reversal of endothelial dysfunction via activation of eNOS (endothelial nitric oxide synthase), and (2) increase of prostacyclin.
In research conducted at the US NIH, cicletanine has been demonstrated as an inhibitor of protein kinase C and marinobufagenin.[3]
References
[edit]- ^ Sassard J (1992). Genetic Hypertension. John Libbey Eurotext. ISBN 978-0-86196-313-3.
- ^ Bagrov AY, Dmitrieva RI, Dorofeeva NA, Fedorova OV, Lopatin DA, Lakatta EG, Droy-Lefaix MT (February 2000). "Cicletanine reverses vasoconstriction induced by the endogenous sodium pump ligand, marinobufagenin, via a protein kinase C dependent mechanism". Journal of Hypertension. 18 (2): 209–215. doi:10.1097/00004872-200018020-00012. PMID 10694190. S2CID 35374482.
- ^ Fedorova OV, Talan MI, Agalakova NI, Droy-Lefaix MT, Lakatta EG, Bagrov AY (March 2003). "Myocardial PKC beta2 and the sensitivity of Na/K-ATPase to marinobufagenin are reduced by cicletanine in Dahl hypertension". Hypertension. 41 (3): 505–511. doi:10.1161/01.HYP.0000053446.43894.9F. PMID 12623951.
