Carbon monoxide-releasing molecules
Carbon monoxide-releasing molecules (CORMs) are chemical compounds designed to release controlled amounts of carbon monoxide (CO). CORMs are being developed as potential therapeutic agents to locally deliver CO to cells and tissues, thus overcoming limitations of CO gas inhalation protocols.
CO is best known for its toxicity in carbon monoxide poisoning at high doses. However, CO is a gasotransmitter and supplemental low dosage of CO has been linked to therapeutic benefits. Pre-clinical research has focused on CO's anti-inflammatory activity with significant applications in cardiovascular disease, oncology, transplant surgery, and neuroprotection.[1]
History
[edit]Therapeutic interest in CO dates back to the study of factitious airs (hydrocarbonate) in the 1790s by Thomas Beddoes, James Watt, James Lind, Humphry Davy, Tiberius Cavallo and many others.[2]
Nickel tetracarbonyl was the first carbonyl-complex used to achieve local delivery of CO and was the first CO delivery molecule suggested to have therapeutic potential in 1891.[2] The acronym CORM was coined in 2002, which marks the first modern biomedical and pharmaceutical initiative.[3] The enzymatic reaction of heme oxygenase inspired the development of synthetic CORMs.
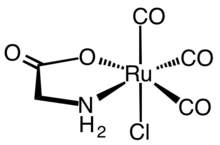
The first synthetic CORMs were typically metal carbonyl complexes. A representative CORM that has been extensively characterized both from a biochemical and pharmacological view point is the ruthenium(II) complex Ru(glycinate)Cl(CO)3, known as CORM-3. Therapeutic data pertaining to metallic CORMs were reappraised to explore if observed effects are due to CO or if metal reactivity mediates physiological effects via thiol depletion, facilitating reduction, ion channel blockage, or redox catalysis.[4][5]
CORM classifications
[edit]Transition metal CORMs
[edit]The majority of therapeutically relevant CORMs are transition metal complexes primarily based on iron, molybdenum, ruthenium, manganese, cobalt, and rhenium.[6]
PhotoCORMs
[edit]The release of CO from carrier agents can be induced photochemically. These carriers are called photoCORMs and include both metal complexes and metal-free (organic) compounds of various structural motifs classified as a special type of photolabile protecting group.[7]
ET-CORMs
[edit]Enzyme-triggered CORMs (ET-CORMs) have been developed to improve selective local delivery of CO. Some ET-CORM prodrugs are activated by esterase enzymes for site specific liberation of CO.[8]
CO prodrugs
[edit]Methylene chloride was the first organic CORM orally administered based on previous reports of carboxyhemoglobin formation via metabolism. The second organic CORM, CORM-A1 (sodium boranocarbonate), was developed based on a 1960s report of CO release from potassium boranocarbonate.[2]
In 2003, cyclic oxocarbons were suggested as a source for therapeutic CO including deltic acid, squaric acid, croconic acid, and rhodizonic acid and their salts.[9]
Enzyme hybrids
[edit]Based on the synergism of the heme oxygenase system and CO delivery, a molecular hybrid-CORM (HYCO) class emerged consisting of a conjoined HO-1 inducer and CORM species. One such HYCO includes a dimethyl fumarate moiety which activates NRF2 to thereby induce HO-1, whilst the CORM moiety also liberates CO.[10]
Carbon monoxide releasing materials
[edit]Carbon monoxide releasing materials (CORMAs) are novel drug formulations and drug delivery platforms which have emerged to overcome the pharmaceutical limitations of most CORM species.[11] Some CORMA consist of a formulation of micelles prepared from triblock copolymers with a CORM entity, which is triggered for release via addition of cysteine. Other CO-releasing scaffolds include polymers, peptides, silica nanoparticles, nanodiamond, magnetic nanoparticles, nanofiber gel, metallodendrimers, and CORM-protein (macromolecule) conjugates.[12][13]
Other advanced drug delivery devices, such as encapsulated CORMs and extracorporeal membrane-inspired technologies, have been developed.[5]
Carboxyhemoglobin infusion
[edit]Carboxyhemoglobin can be infused to deliver CO. The most common approaches are based on polyethylene glycol PEGylated bovine carboxyhemoglobin and maleimide PEG conjugated human carboxyhemoglobin.[14]
Porphyrins
[edit]Porphyrin structures such as heme, hemin, and metallic protoporphyrin IX (PPIX) analogs (such as cobalt PPIX) have been deployed to induce heme oxygenase and subsequently undergo biotransformation to liberate CO, the inorganic ion, and biliverdin/bilirubin.[15] Some PPIX analogs such as tin PPIX, tin mesoporphyrin, and zinc PPIX, are heme oxygenase inhibitors.
Endogenous CO
[edit]HMOX is the main source of endogenous CO production, though other minor contributors have also been identified.[16] CO is formed at a rate of 16.4 μmol/hour in the human body, ~86% originating from heme via heme oxygenase and ~14% from non-heme sources including: photooxidation, lipid peroxidation, and xenobiotics.[17] The average carboxyhemoglobin (CO-Hb) level in a non-smoker is under 3% CO-Hb (whereas a smoker may reach levels near 10% CO-Hb),[18] though geographic location, occupation, health and behavior are contributing variables.
Heme oxygenase
[edit]In the late 1960s Rudi Schmid characterized the enzyme that facilitates the reaction for heme catabolism, thereby identifying the heme oxygenase (HMOX) enzyme.
HMOX is a heme-containing member of the heat shock protein (HSP) family identified as HSP32. Three isoforms of HMOX have been identified to date including the stress-induced HMOX-1 and constitutive HMOX-2. HMOX-1 is considered a cell rescue protein which is induced in response to oxidative stress and numerous disease states. Furthermore, HMOX-1 is induced by countless molecules including statins, hemin, and natural products.[19][20]
HMOX catalyzes the degradation of heme to biliverdin/bilirubin, ferrous ion, and CO. Though present throughout the body, HO has significant activity in the spleen in the degradation of hemoglobin during erythrocyte recycling (0.8% of the erythrocyte pool per day), which accounts for ~80% of heme derived endogenous CO production. The majority of the remaining 20% of heme derived CO production is attributed to hepatic catabolism of hemoproteins (myoglobin, cytochromes, catalase, peroxidases, soluble guanylate cyclase, nitric oxide synthase) and ineffective erythropoiesis in bone marrow.[21]
The enzymatic velocity and catalytic activity of HMOX can be enhanced by a plethora of dietary substances and xenobiotics to increase CO production.
Minor CO sources
[edit]The formation of CO from lipid peroxidation was first reported in the late 1960s and is regarded as a minor contributor to endogenous CO production.[22][23] Other contributing sources include: the microbiome, cytochrome P450 reductase, human acireductone dioxygenase, tyrosinase, lipid peroxidation, alpha-keto acids, and other oxidative and redox mechanisms.[16]
CO pharmacology
[edit]Carbon monoxide is one of three gaseous signaling molecules alongside nitric oxide and hydrogen sulfide. These gases are collectively referred to as gasotransmitters. CO is a classical example of hormesis such that low-dose is essential and beneficial, whereas an absence or excessive exposure to CO can be toxic.
Signaling
[edit]The first evidence of CO as a signaling molecule occurred upon observation of CO stimulating soluble guanylate cyclase and subsequent cyclic guanosine monophosphate (cGMP) production to serve as a vasodilator in vascular smooth muscle cells. The anti-inflammatory effects of CO are attributed to activation of the p38 mitogen-activated protein kinase (MAPK) pathway. While CO commonly interacts with the ferrous iron atom of heme in a hemoprotein,[24] it has been demonstrated that CO activates calcium-dependent potassium channels by engaging in hydrogen-bonding with surface histidine residues.[16][25]
CO may have an inhibitory effect on numerous proteins including cytochrome P450 and cytochrome c oxidase.[26]
Pharmacokinetics
[edit]CO has approximately 210x greater affinity for hemoglobin than oxygen. The equilibrium dissociation constant for the reaction Hb-CO ⇌ Hb + CO strongly favours the CO complex, thus the release of CO for pulmonary excretion generally takes some time.
CO is considered non-reactive in the body and primarily undergoes pulmonary excretion.[27]
References
[edit]- ^ Motterlini R, Otterbein LE (September 2010). "The therapeutic potential of carbon monoxide". review article. Nature Reviews. Drug Discovery. 9 (9): 728–743. doi:10.1038/nrd3228. PMID 20811383. S2CID 205477130.
- ^ a b c Hopper CP, Zambrana PN, Goebel U, Wollborn J (June 2021). "A brief history of carbon monoxide and its therapeutic origins". Nitric Oxide. 111–112: 45–63. doi:10.1016/j.niox.2021.04.001. PMID 33838343. S2CID 233205099.
- ^ Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ (February 2002). "Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities". Circulation Research. 90 (2): E17–E24. doi:10.1161/hh0202.104530. PMID 11834719. S2CID 12515186.
- ^ Southam HM, Smith TW, Lyon RL, Liao C, Trevitt CR, Middlemiss LA, et al. (September 2018). "A thiol-reactive Ru(II) ion, not CO release, underlies the potent antimicrobial and cytotoxic properties of CO-releasing molecule-3". Redox Biology. 18: 114–123. doi:10.1016/j.redox.2018.06.008. PMC 6067063. PMID 30007887.
- ^ a b Yang X, Lu W, Wang M, Tan C, Wang B (October 2021). ""CO in a pill": Towards oral delivery of carbon monoxide for therapeutic applications". Journal of Controlled Release. 338: 593–609. doi:10.1016/j.jconrel.2021.08.059. PMC 8526413. PMID 34481027.
- ^ Schatzschneider U (March 2015). "Novel lead structures and activation mechanisms for CO-releasing molecules (CORMs)". British Journal of Pharmacology. 172 (6): 1638–1650. doi:10.1111/bph.12688. PMC 4369270. PMID 24628281.
- ^ Weinstain R, Slanina T, Kand D, Klán P (December 2020). "Visible-to-NIR-Light Activated Release: From Small Molecules to Nanomaterials". Chemical Reviews. 120 (24): 13135–13272. doi:10.1021/acs.chemrev.0c00663. PMC 7833475. PMID 33125209.
- ^ Stamellou E, Storz D, Botov S, Ntasis E, Wedel J, Sollazzo S, et al. (2014). "Different design of enzyme-triggered CO-releasing molecules (ET-CORMs) reveals quantitative differences in biological activities in terms of toxicity and inflammation". Redox Biology. 2: 739–748. doi:10.1016/j.redox.2014.06.002. PMC 4085349. PMID 25009775.
- ^ Alberto R, Motterlini R (May 2007). "Chemistry and biological activities of CO-releasing molecules (CORMs) and transition metal complexes". Dalton Transactions (17): 1651–1660. doi:10.1039/b701992k. PMID 17443255.
- ^ Pol O (January 2021). "The role of carbon monoxide, heme oxygenase 1, and the Nrf2 transcription factor in the modulation of chronic pain and their interactions with opioids and cannabinoids". Medicinal Research Reviews. 41 (1): 136–155. doi:10.1002/med.21726. PMID 32820550. S2CID 221219782.
- ^ Heinemann SH, Hoshi T, Westerhausen M, Schiller A (April 2014). "Carbon monoxide--physiology, detection and controlled release". review article. Chemical Communications. 50 (28): 3644–3660. doi:10.1039/c3cc49196j. PMC 4072318. PMID 24556640.
- ^ Nguyen D, Boyer C (October 2015). "Macromolecular and Inorganic Nanomaterials Scaffolds for Carbon Monoxide Delivery: Recent Developments and Future Trends". review article. ACS Biomaterials Science & Engineering. 1 (10): 895–913. doi:10.1021/acsbiomaterials.5b00230. PMID 33429521.
- ^ Kautz AC, Kunz PC, Janiak C (November 2016). "CO-releasing molecule (CORM) conjugate systems". review article. Dalton Transactions. 45 (45): 18045–18063. doi:10.1039/c6dt03515a. PMID 27808304.
- ^ Hopper CP, Meinel L, Steiger C, Otterbein LE (July 2018). "Where is the Clinical Breakthrough of Heme Oxygenase-1 / Carbon Monoxide Therapeutics?". Current Pharmaceutical Design. 24 (20): 2264–2282. doi:10.2174/1381612824666180723161811. PMID 30039755. S2CID 51712930.
- ^ Maines MD (July 1988). "Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications". review article. FASEB Journal. 2 (10): 2557–2568. doi:10.1096/fasebj.2.10.3290025. PMID 3290025. S2CID 22652094.
- ^ a b c Hopper CP, De La Cruz LK, Lyles KV, Wareham LK, Gilbert JA, Eichenbaum Z, et al. (December 2020). "Role of Carbon Monoxide in Host-Gut Microbiome Communication". Chemical Reviews. 120 (24): 13273–13311. doi:10.1021/acs.chemrev.0c00586. PMID 33089988. S2CID 224824871.
- ^ Wang R, ed. (2001). Carbon monoxide and cardiovascular functions. review article (first ed.). CRC Press. p. 5. ISBN 978-1-4200-4101-9.
- ^ Thom SR (2008). "Chapter 15: Carbon monoxide pathophysiology and treatment". In Neuman TS, Thom SR (eds.). Physiology and medicine of hyperbaric oxygen therapy. review article. pp. 321–347. doi:10.1016/B978-1-4160-3406-3.50020-2. ISBN 978-1-4160-3406-3.
- ^ Correa-Costa M, Otterbein LE (2014). "Eat to Heal: Natural Inducers of the Heme Oxygenase-1 System.". In Folkerts G, Garssen J (eds.). Pharma-Nutrition. review article. AAPS Advances in the Pharmaceutical Sciences Series. Vol. 12. Springer, Cham. pp. 243–256. doi:10.1007/978-3-319-06151-1_12. ISBN 978-3-319-06150-4.
- ^ Ferrándiz ML, Devesa I (2008). "Inducers of heme oxygenase-1". review article. Current Pharmaceutical Design. 14 (5): 473–486. doi:10.2174/138161208783597399. PMID 18289074.
- ^ Breman HJ, Wong RJ, Stevenson DK (30 October 2001). "Chapter 15: Sources, Sinks, and Measurement of Carbon Monoxide". In Wang R (ed.). Carbon Monoxide and Cardiovascular Functions. review article (2nd ed.). CRC Press. ISBN 978-0-8493-1041-6.
- ^ Wolff DG (December 1976). "The formation of carbon monoxide during peroxidation of microsomal lipids". primary article. Biochemical and Biophysical Research Communications. 73 (4): 850–857. doi:10.1016/0006-291X(76)90199-6. PMID 15625852.
- ^ Nishibayashi H, Omma T, Sato R, Estabrook RW, Okunuki K, Kamen MD, Sekuzu I, eds. (1968). Structure and Function of Cytochromes. review article. University Park Press. pp. 658–665.
- ^ Motterlini R, Foresti R (March 2017). "Biological signaling by carbon monoxide and carbon monoxide-releasing molecules". American Journal of Physiology. Cell Physiology. 312 (3): C302–C313. doi:10.1152/ajpcell.00360.2016. PMID 28077358. S2CID 21861993.
- ^ Wilkinson WJ, Kemp PJ (July 2011). "Carbon monoxide: an emerging regulator of ion channels". review article. The Journal of Physiology. 589 (Pt 13): 3055–3062. doi:10.1113/jphysiol.2011.206706. PMC 3145923. PMID 21521759.
- ^ Correia MA, Ortiz de Montellano PR (2005). "Inhibition of cytochrome P450 enzymes". Cytochrome P450. review article. Boston, MA: Springer. pp. 247–322. doi:10.1007/0-387-27447-2_7. ISBN 978-0-306-48324-0.
- ^ Wilbur S, Williams M, Williams R, Scinicariello F, Klotzbach JM, Diamond GL, Citra M (2012). "Health Effects". Toxicological Profile for Carbon Monoxide. review article. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. PMID 23946966.
Further reading
[edit]- Kim HH, Choi S (August 2018). "Therapeutic Aspects of Carbon Monoxide in Cardiovascular Disease". review article. International Journal of Molecular Sciences. 19 (8): 2381. doi:10.3390/ijms19082381. PMC 6121498. PMID 30104479.
- Ismailova A, Kuter D, Bohle DS, Butler IS (2018). "An Overview of the Potential Therapeutic Applications of CO-Releasing Molecules". review article. Bioinorganic Chemistry and Applications. 2018: 8547364. doi:10.1155/2018/8547364. PMC 6109489. PMID 30158958.
- Abeyrathna N, Washington K, Bashur C, Liao Y (October 2017). "Nonmetallic carbon monoxide releasing molecules (CORMs)". review article. Organic & Biomolecular Chemistry. 15 (41): 8692–8699. doi:10.1039/c7ob01674c. PMID 28948260.
- Hopper CP, Wollborn J (August 2019). "Delivery of carbon monoxide via halogenated ether anesthetics". review article. Nitric Oxide. 89: 93–95. doi:10.1016/j.niox.2019.05.006. PMID 31125687. S2CID 164217698.
- Wilson JL, Jesse HE, Poole RK, Davidge KS (May 2012). "Antibacterial effects of carbon monoxide". review article. Current Pharmaceutical Biotechnology. 13 (6): 760–768. doi:10.2174/138920112800399329. PMID 22201612.
- Slanina T, Šebej P (June 2018). "Visible-light-activated photoCORMs: rational design of CO-releasing organic molecules absorbing in the tissue-transparent window". review article. Photochemical & Photobiological Sciences. 17 (6): 692–710. doi:10.1039/C8PP00096D. PMID 29796556.
