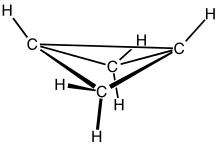
Bicyclobutane
| |
| Names | |
|---|---|
| Preferred IUPAC name
Bicyclo[1.1.0]butane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6 | |
| Molar mass | 54.092 g·mol−1 |
| Appearance | colorless gas |
| Boiling point | 8.3 ± 0.2 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bicyclo[1.1.0]butane is an organic compound with the formula C4H6. It is a bicyclic molecule consisting of two cis-fused cyclopropane rings, and is a colorless and easily condensed gas.[1] Bicyclobutane is noted for being one of the most strained compounds that is isolatable on a large scale — its strain energy is estimated at 63.9 kcal mol−1. It is a nonplanar molecule, with a dihedral angle between the two cyclopropane rings of 123°.[2]
The first reported bicyclobutane was the ethyl carboxylate derivative, C4H5CO2Et, which was prepared by dehydrohalogenation the corresponding bromocyclobutanecarboxylate ester with sodium hydride.[2] The parent hydrocarbon was prepared from 1-bromo-3-chlorocyclobutane by conversion of the bromocyclobutanecarboxylate ester,[1] followed by intramolecular Wurtz coupling using molten sodium.[3] The intermediate 1-bromo-3-chlorocyclobutane can also be prepared via a modified Hunsdiecker reaction from 3-chlorocyclobutanecarboxylic acid using mercuric oxide and bromine:[4]
A synthetic approach to bicyclobutane derivatives involves ring closure of a suitably substituted 2-bromo-1-(chloromethyl)cyclopropane with magnesium in THF.[5] Substituted bicyclo[1.1.0]butanes can also be prepared from the reaction of iodo-bicyclo[1.1.1]pentanes with amines, thiols, and sulfinate salts.[6] Bicyclo[1.1.0]butanes are explored in medicinal chemistry as covalent reactive groups.[7]
Stereochemical evidence indicates that bicyclobutane undergoes thermolysis to form 1,3-butadiene with an activation energy of 41 kcal mol−1 via a concerted pericyclic mechanism (cycloelimination, [σ2s+σ2a]).[8]
Biological synthesis
[edit]Linolenic acid can be converted into its bicyclobutane derivative using a fusion protein produced by a strain of the cyanobacterium Anabaena sphaerica (strain PCC 7120).[9] The other group reported a directed evolution approach, whereby engineered heme protein was expressed in E. coli and optimized for rate and yield of a substituted bicyclobutane derivative.[10]
See also
[edit]- Propalene (Bicyclobutadiene)
- Bicyclopentane
- 1.1.1-Propellane
References
[edit]- ^ a b Wiberg, K. B.; Lampman, G. M.; Ciula, R. P.; Connor, D. S.; Schertler, P.; Lavanish, J. (1965). "Bicyclo[1.1.0]butane". Tetrahedron. 21 (10): 2749–2769. doi:10.1016/S0040-4020(01)98361-9.
- ^ a b Wiberg, K. B. (1968). "Small Ring Bicyclo[n.m.0]alkanes". In Hart, H.; Karabatsos, G. J. (eds.). Advances in Alicyclic Chemistry. Vol. 2. Academic Press. pp. 185–254. ISBN 9781483224213.
- ^ Lampman, Gary M.; Aumiller, James C. (1971). "Bicyclo[1.1.0]butane". Organic Syntheses. 51: 55. doi:10.15227/orgsyn.051.0055.
- ^ Lampman, Gary M.; Aumiller, James C. (1971). "Mercury(II) oxide-modified Hunsdiecker reaction: 1-Bromo-3-chlorocyclobutane". Organic Syntheses. 51: 106. doi:10.15227/orgsyn.051.0106.
- ^ D'yachenko, A. I.; Abramova, N. M.; Zotova, S. V.; Nesmeyanova, O. A.; Bragin, O. V. (1985). "New synthesis of bicyclo[1.1.0]butane hydrocarbons". Bulletin of the Academy of Sciences of the USSR. 34 (9): 1885–1889. doi:10.1007/BF00953929. S2CID 96988412.
- ^ Mandler, Michael D.; Mignone, James; Jurica, Elizabeth A.; Palkowitz, Maximilian D.; Aulakh, Darpandeep; Cauley, Anthony N.; Farley, Christopher A.; Zhang, Shasha; Traeger, Sarah C.; Sarjeant, Amy; Paiva, Anthony; Perez, Heidi L.; Ellsworth, Bruce A.; Regueiro-Ren, Alicia (10 November 2023). "Synthesis of Bicyclo[1.1.0]butanes from Iodo-Bicyclo[1.1.1]pentanes". Organic Letters. 25 (44). American Chemical Society: 7947–7952. doi:10.1021/acs.orglett.3c01417. ISSN 1523-7060.
- ^ Tokunaga, Keisuke; Sato, Mami; Kuwata, Keiko; Miura, Chizuru; Fuchida, Hirokazu; Matsunaga, Naoya; Koyanagi, Satoru; Ohdo, Shigehiro; Shindo, Naoya; Ojida, Akio (2020-10-28). "Bicyclobutane Carboxylic Amide as a Cysteine-Directed Strained Electrophile for Selective Targeting of Proteins". Journal of the American Chemical Society. 142 (43): 18522–18531. doi:10.1021/jacs.0c07490. ISSN 0002-7863. PMID 33047956.
- ^ Woodward, Robert B.; Hoffmann, Roald (1969). "The Conservation of Orbital Symmetry". Angewandte Chemie International Edition. 8 (11): 781–853. doi:10.1002/anie.196907811.
- ^ Schneider, Claus; Niisuke, Katrin; Boeglin, William E.; Voehler, Markus; Stec, Donald F.; Porter, Ned A.; Brash, Alan R. (2007-11-27). "Enzymatic synthesis of a bicyclobutane fatty acid by a hemoprotein lipoxygenase fusion protein from the cyanobacterium Anabaena PCC 7120". Proceedings of the National Academy of Sciences of the United States of America. 104 (48): 18941–18945. Bibcode:2007PNAS..10418941S. doi:10.1073/pnas.0707148104. ISSN 1091-6490. PMC 2141887. PMID 18025466.
- ^ Chen, Kai; Huang, Xiongyi; Kan, S. B. Jennifer; Zhang, Ruijie K.; Arnold, Frances H. (6 April 2018). "Enzymatic construction of highly strained carbocycles". Science. 360 (6384): 71–75. Bibcode:2018Sci...360...71C. doi:10.1126/science.aar4239. ISSN 1095-9203. PMC 6104391. PMID 29622650.

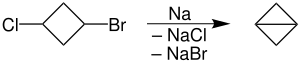
![Bicyclo[1.1.1]pentanes to Bicyclo[1.1.0]butanes](http://upload.wikimedia.org/wikipedia/commons/thumb/d/da/SchemeBCBs.svg/582px-SchemeBCBs.svg.png)