Bephenium hydroxynaphthoate
Appearance
(Redirected from Bephenium)
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | <1% |
| Excretion | Renal (negligible) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.021.189 |
| Chemical and physical data | |
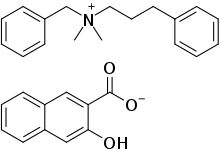
| Formula | C28H29NO4 |
| Molar mass | 443.543 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bephenium hydroxynaphthoate (INN, trade names Alcopara, Alcopar, Befenium, Debefenium, Francin, Nemex) is an anthelmintic agent formerly used in the treatment of hookworm infections and ascariasis.[1][2] It is formulated as a salt between the active pharmaceutical ingredient, bephenium, and 3-hydroxy-2-naphthoic acid.[citation needed]
Bephenium is not FDA-approved and is not available in the United States.[3]
References
[edit]- ^ Sweetman S, ed. (2009). Martindale: The complete drug reference (36th ed.). London: Pharmaceutical Press. p. 143. ISBN 978-0-85369-840-1.
- ^ Jayewardene G, Ismail MM, Wijayaratnam Y (July 1960). "Bephenium hydroxynaphthoate in treatment of ascariasis". Br Med J. 2 (5194): 268–71. doi:10.1136/bmj.2.5194.268. PMC 2097409. PMID 14406934.
- ^ Pham PA (March 19, 2009). "Bephenium hydroxynaphthoate". Point-of-Care Information Technology ABX Guide. Johns Hopkins University. Retrieved on March 25, 2011.
