Hippocampus anatomy


Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In the human and other primates, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.
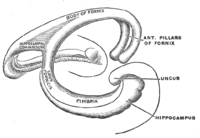
Topologically, the surface of a cerebral hemisphere can be regarded as a sphere with an indentation where it attaches to the midbrain. The structures that line the edge of the hole collectively make up the so-called limbic system (Latin limbus = border), with the hippocampus lining the posterior edge of this hole. These limbic structures include the hippocampus, cingulate cortex, olfactory cortex, and amygdala. Paul MacLean once suggested, as part of his triune brain theory, that the limbic structures constitute the neural basis of emotion. While most neuroscientists no longer believe in the concept of a unified "limbic system", these regions are highly interconnected and do interact with one another.[citation needed]
Basic hippocampal circuit
[edit]
Starting at the dentate gyrus and working inward along the S-curve of the hippocampus means traversing a series of narrow zones. The first of these, the dentate gyrus (DG), is actually a separate structure, a tightly packed layer of small granule cells wrapped around the end of the hippocampus proper, forming a pointed wedge in some cross-sections, a semicircle in others. Next come a series of Cornu Ammonis areas: first CA4 (which underlies the dentate gyrus), then CA3, then a very small zone called CA2, then CA1. The CA areas are all filled with densely packed pyramidal cells similar to those found in the neocortex. After CA1 comes an area called the subiculum. After this comes a pair of ill-defined areas called the presubiculum and parasubiculum, then a transition to the cortex proper (mostly the entorhinal area of the cortex). Most anatomists use the term "hippocampus proper" to refer to the four CA fields, and hippocampal formation to refer to the hippocampus proper plus dentate gyrus and subiculum.[1]
The major signaling pathways flow through the hippocampus and combine to form a loop. Most external input comes from the adjoining entorhinal cortex, via the axons of the so-called perforant path. These axons arise from layer 2 of the entorhinal cortex (EC), and terminate in the dentate gyrus and CA3. There is also a distinct pathway from layer 3 of the EC directly to CA1, often referred to as the temporoammonic or TA-CA1 pathway. Granule cells of the DG send their axons (called "mossy fibers") to CA3. Pyramidal cells of CA3 send their axons to CA1. Pyramidal cells of CA1 send their axons to the subiculum and deep layers of the EC. Subicular neurons send their axons mainly to the EC. The perforant path-to-dentate gyrus-to-CA3-to-CA1 was called the trisynaptic circuit by Per Andersen, who noted that thin slices could be cut out of the hippocampus perpendicular to its long axis, in a way that preserves all of these connections. This observation was the basis of his lamellar hypothesis, which proposed that the hippocampus can be thought of as a series of parallel strips, operating in a functionally independent way.[2] The lamellar concept is still sometimes considered to be a useful organizing principle, but more recent data, showing extensive longitudinal connections within the hippocampal system, have required it to be substantially modified.[3]
Perforant path input from EC layer II enters the dentate gyrus and is relayed to region CA3 (and to mossy cells, located in the hilus of the dentate gyrus, which then send information to distant portions of the dentate gyrus where the cycle is repeated). Region CA3 combines this input with signals from EC layer II and sends extensive connections within the region and also sends connections to strata radiatum and oriens of ipsilateral and contralateral CA1 regions through a set of fibers called the Schaffer collaterals, and commissural pathway, respectively.[4][5][6] Region CA1 receives input from the CA3 subfield, EC layer III and the nucleus reuniens of the thalamus (which project only to the terminal apical dendritic tufts in the stratum lacunosum-moleculare). In turn, CA1 projects to the subiculum as well as sending information along the aforementioned output paths of the hippocampus. The subiculum is the final stage in the pathway, combining information from the CA1 projection and EC layer III to also send information along the output pathways of the hippocampus.
The hippocampus also receives a number of subcortical inputs. In Macaca fascicularis, these inputs include the amygdala (specifically the anterior amygdaloid area, the basolateral nucleus, and the periamygdaloid cortex), the medial septum and the diagonal band of Broca, the claustrum, the substantia innominata and the basal nucleus of Meynert, the thalamus (including the anterior nuclear complex, the laterodorsal nucleus, the paraventricular and parataenial nuclei, the nucleus reuniens, and the nucleus centralis medialis), the lateral preoptic and lateral hypothalamic areas, the supramammillary and retromammillary regions, the ventral tegmental area, the tegmental reticular fields, the raphe nuclei (the nucleus centralis superior and the dorsal raphe nucleus), the nucleus reticularis tegementi pontis, the periaqueductal gray, the dorsal tegmental nucleus, and the locus coeruleus. The hippocampus also receives direct monosynaptic projections from the cerebellar fastigial nucleus.[7]
Major fiber systems in the rat
[edit]Angular bundle
[edit]These fibers start from the ventral part of entorhinal cortex (EC) and contain commissural (EC◀▶Hippocampus) and Perforant path (excitatory EC▶CA1, and inhibitory EC◀▶CA2[8]) fibers. They travel along the septotemporal axis of the hippocampus. Perforant path fibers, as the name suggests, perforate subiculum before going to the hippocampus (CA fields) and dentate gyrus.[9]
Fimbria-fornix pathway
[edit]
Fimbria-fornix fibers are the hippocampal and subicular gateway to and from subcortical brain regions.[10][11] Different parts of this system are given different names:
- White myelinated fibers that cover the ventricular (deep) parts of hippocampus make alveus.
- Fibers that cover the temporal parts of hippocampus make a fiber bundle that is called fimbria. Going from temporal to septal (dorsal) parts of hippocampus fimbria collects more and more hippocampal and subicular outputs and becomes thicker.
- In the midline and under the corpus callosum, these fibers form the fornix.
At the circuit level, the alveus contains axonal fibers from the DG and from Pyramidal neurons of CA3, CA2, CA1 and subiculum (CA1 ▶ subiculum and CA1 ▶ entorhinal projections) that collect in the temporal hippocampus to form the fimbria/fornix, one of the major outputs of the hippocampus.[12][13][14][15][16] In the rat, some medial and lateral entorhinal axons (entorhinal ▶ CA1 projection) pass through alveus towards the CA1 stratum lacunosum moleculare without making a significant number of en passant boutons on other CA1 layers (Temporoammonic alvear pathway).[13][17] Contralateral entorhinal ▶ CA1 projections almost exclusively pass through alveus. The more septal the more ipsilateral entorhinal-CA1 projections that take alvear pathway (instead of perforant path).[18] Although subiculum sends axonal projections to alveus, subiculum ▶ CA1 projection passes through strata oriens and moleculare of subiculum and CA1.[19] Cholinergic and GABAergic projections from MS-DBB to CA1 also pass through Fimbria.[20] Fimbria stimulation leads to cholinergic excitation of CA1 O-LMR cells.[21]
It is also known that extracellular stimulation of fimbria stimulates CA3 Pyramidal cells antidromically and orthodromically, but it has no impact on dentate granule cells.[22] Each CA1 Pyramidal cell also sends an axonal branch to fimbria.[23][24]
Hippocampal commissures
[edit]Hilar mossy cells and CA3 Pyramidal cells are the main origins of hippocampal commissural fibers. They pass through hippocampal commissures to reach contralateral regions of hippocampus. Hippocampal commissures have dorsal and ventral segments. Dorsal commissural fibers consists mainly of entorhinal and presubicular fibers to or from the hippocampus and dentate gyrus.[9] As a rule of thumb, one could say that each cytoarchitectonic field that contributes to the commissural projection also has a parallel associational fiber that terminates in the ipsilateral hippocampus.[25] The inner molecular layer of dentate gyrus (dendrites of both granule cells and GABAergic interneurons) receives a projection that has both associational and commissural fibers mainly from hilar mossy cells and to some extent from CA3c Pyramidal cells. Because this projection fibers originate from both ipsilateral and contralateral sides of hippocampus they are called associational/commissural projections. In fact, each mossy cell innervates both the ipsilateral and contralateral dentate gyrus. The well known trisynaptic circuit of the hippocampus spans mainly horizontally along the hippocampus. However, associational/commissural fibers, like CA2 Pyramidal cell associational projections, span mainly longitudinally (dorsoventrally) along the hippocampus.[26][27] Commissural fibers that originate from CA3 Pyramidal cells go to CA3, CA2 and CA1 regions. Like mossy cells, a single CA3 Pyramidal cell contributes to both commissural and associational fibers, and they terminate on both principal cells and interneurons.[28][29] A weak commissural projection connects both CA1 regions together. Subiculum has no commissural inputs or outputs. In comparison with rodents, hippocampal commissural connections are much less abundant in the monkey and humans.[30] Although excitatory cells are the main contributors to commissural pathways, a GABAergic component has been reported among their terminals which were traced back to hilus as origin.[31] Stimulation of commissural fibers stimulates DG hilar perforant path-associated (HIPP) and CA3 trilaminar cells antidromically.[32]
Hippocampal cells and layers
[edit]

Hippocampus proper
[edit]The hippocampus proper is composed of a number of subfields. Though terminology varies among authors, the terms most frequently used are dentate gyrus and the cornu ammonis (literally "Ammon's horn", abbreviated CA). The dentate gyrus contains the fascia dentata and the hilus, while the CA is differentiated into subfields CA1, CA2, CA3, and CA4.
However, the region known as CA4 is in fact the "deep, polymorphic layer of the dentate gyrus"[33] (as clarified by Theodor Blackstad (1956)[34] and by David Amaral (1978)).[35]
Cut in cross section, the hippocampus is a C-shaped structure that resembles a ram's horns. The name cornu ammonis refers to the Egyptian deity Amun, who has the head of a ram. The horned appearance of the hippocampus is caused by cell density differentials and varying degrees of neuronal fibers.
In rodents, the hippocampus is positioned so that, roughly, one end is near the top of the head (the dorsal or septal end) and one end near the bottom of the head (the ventral or temporal end). As shown in the figure, the structure itself is curved and subfields or regions are defined along the curve, from CA4 through CA1 (only CA3 and CA1 are labeled). The CA regions are also structured depthwise in clearly defined strata (or layers):
- Stratum oriens (str. oriens) is the next layer superficial to the alveus. The cell bodies of inhibitory basket cells and horizontal trilaminar cells, named for their axons innervating three layers—the oriens, Pyramidal, and radiatum are located in this stratum. The basal dendrites of Pyramidal neurons are also found here, where they receive input from other Pyramidal cells, septal fibers and commissural fibers from the contralateral hippocampus (usually recurrent connections, especially in CA3 and CA2.) In rodents the two hippocampi are highly connected, but in primates this commissural connection is much sparser.
- Stratum pyramidale (str. pyr.) contains the cell bodies of the Pyramidal neurons, which are the principal excitatory neurons of the hippocampus. This stratum tends to be one of the more visible strata to the naked eye. In region CA3, this stratum contains synapses from the mossy fibers that course through stratum lucidum. This stratum also contains the cell bodies of many interneurons, including axo-axonic cells, bistratified cells, and radial trilaminar cells.
- Stratum lucidum (str. luc.) is one of the thinnest strata in the hippocampus and only found in the CA3 region. Mossy fibers from the dentate gyrus granule cells course through this stratum in CA3, though synapses from these fibers can be found in str. pyr.
- Stratum radiatum (str. rad.), like str. oriens, contains septal and commissural fibers. It also contains Schaffer collateral fibers, which are the projection forward from CA3 to CA1. Some interneurons that can be found in more superficial layers can also be found here, including basket cells, bistratified cells, and radial trilaminar cells.
- Stratum lacunosum (str. lac.) is a thin stratum that too contains Schaffer collateral fibers, but it also contains perforant path fibers from the superficial layers of entorhinal cortex. Due to its small size, it is often grouped together with stratum moleculare into a single stratum called stratum lacunosum-moleculare (str. l-m.).
- Stratum moleculare (str. mol.) is the most superficial stratum in the hippocampus. Here the perforant path fibers form synapses onto the distal, apical dendrites of Pyramidal cells.
- Hippocampal sulcus (sulc.) or fissure is a cell-free region that separates the CA1 field from the dentate gyrus. Because the phase of recorded theta rhythm varies systematically through the strata, the sulcus is often used as a fixed reference point for recording EEG as it is easily identifiable.[33]
Dentate gyrus
[edit]The dentate gyrus is composed of a similar series of strata:
- The polymorphic layer (poly. lay.) is the most superficial layer of the dentate gyrus and is often considered a separate subfield (as the hilus). This layer contains many interneurons, and the axons of the dentate granule cells pass through this stratum on the way to CA3.
- Stratum granulosum (str. gr.) contains the cell bodies of the dentate granule cells.
- Stratum moleculare, inner third (str. mol. 1/3) is where both commissural fibers from the contralateral dentate gyrus run and form synapses as well as where inputs from the medial septum terminate, both on the proximal dendrites of the granule cells.
- Stratum moleculare, external two thirds (str. mol. 2/3) is the deepest of the strata, sitting just superficial to the hippocampal sulcus across from stratum moleculare in the CA fields. The perforant path fibers run through this strata, making excitatory synapses onto the distal apical dendrites of granule cells.
An up-to-date knowledge base of hippocampal formation neuronal types, their biomarker profile, active and passive electrophysiological parameters, and connectivity is supported at the Hippocampome website.[36]
References
[edit]- ^ Amaral, D; Lavenex P (2006). "Ch 3. Hippocampal Neuroanatomy". In Andersen P; Morris R; Amaral D; Bliss T; O'Keefe J (eds.). The Hippocampus Book. Oxford University Press. ISBN 978-0-19-510027-3.
- ^ Andersen, P; Bliss TVP; Skrede KK (1971). "Lamellar organization of hippocampal excitatory pathways". Exp. Brain Res. 13 (2): 222–238. doi:10.1007/BF00234087. PMID 5570425. S2CID 12075886.
- ^ Andersen, P; Soleng AF; Raastad M (2000). "The hippocampal lamella hypothesis revisited". Brain Res. 886 (1–2): 165–171. doi:10.1016/S0006-8993(00)02991-7. PMID 11119694. S2CID 8455285.
- ^ Hjorth-Simonsen, A (15 January 1973). "Some intrinsic connections of the hippocampus in the rat: an experimental analysis". The Journal of Comparative Neurology. 147 (2): 145–61. doi:10.1002/cne.901470202. PMID 4118866. S2CID 28989051.
- ^ Swanson, LW; Wyss, JM; Cowan, WM (15 October 1978). "An autoradiographic study of the organization of intrahippocampal association pathways in the rat". The Journal of Comparative Neurology. 181 (4): 681–715. doi:10.1002/cne.901810402. PMID 690280. S2CID 30954240.
- ^ Laurberg, S (15 April 1979). "Commissural and intrinsic connections of the rat hippocampus". The Journal of Comparative Neurology. 184 (4): 685–708. doi:10.1002/cne.901840405. PMID 422759. S2CID 27256712.
- ^ Heath RG, Harper JW (November 1974). "Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats". Exp. Neurol. 45 (2): 268–87. doi:10.1016/0014-4886(74)90118-6. PMID 4422320.
- ^ Melzer, S.; Michael, M.; Caputi, A.; Eliava, M.; Fuchs, E. C.; Whittington, M. A.; Monyer, H. (22 March 2012). "Long-Range-Projecting GABAergic Neurons Modulate Inhibition in Hippocampus and Entorhinal Cortex". Science. 335 (6075): 1506–1510. Bibcode:2012Sci...335.1506M. doi:10.1126/science.1217139. PMID 22442486. S2CID 206539012.
- ^ a b Andersen, Per; et al., eds. (2007). The hippocampus book. New York: Oxford University Press. p. 47,63,123. ISBN 9780195100273.
- ^ POWELL, TP; GUILLERY, RW; COWAN, WM (October 1957). "A quantitative study of the fornixmamillo-thalamic system". Journal of Anatomy. 91 (4): 419–37. PMC 1244899. PMID 13475143.
- ^ DAITZ, HM; POWELL, TP (February 1954). "Studies of the connexions of the fornix system". Journal of Neurology, Neurosurgery, and Psychiatry. 17 (1): 75–82. doi:10.1136/jnnp.17.1.75. PMC 503161. PMID 13131081.
- ^ Knowles, WD; Schwartzkroin, PA (November 1981). "Axonal ramifications of hippocampal Ca1 Pyramidal cells". The Journal of Neuroscience. 1 (11): 1236–41. doi:10.1523/JNEUROSCI.01-11-01236.1981. PMC 6564220. PMID 6171629.
- ^ a b The hippocampus book. New York: Oxford University Press. 2007. p. 47. ISBN 9780199723164.
- ^ Alloway, Thomas C. Pritchard, Kevin D. (1999). Medical neuroscience (1st ed.). Madison, Conn.: Fence Creek Pub. p. 28. ISBN 978-1889325293.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Gaudron, Henri M. Duvernoy, Françoise Cattin, Pierre-Yves Risold; drawings and illustrations by J.L. Vannson and M. (2013). The human hippocampus functional anatomy, vascularization, and serial sections with MRI (4th ed.). Berlin: Springer. p. 28. ISBN 978-3-642-33603-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Tamamaki, N; Abe, K; Nojyo, Y (14 June 1988). "Three-dimensional analysis of the whole axonal arbors originating from single CA2 Pyramidal neurons in the rat hippocampus with the aid of a computer graphic technique". Brain Research. 452 (1–2): 255–72. doi:10.1016/0006-8993(88)90030-3. PMID 3401733. S2CID 25038544.
- ^ Shetty, AK (2002). "Entorhinal axons exhibit sprouting in CA1 subfield of the adult hippocampus in a rat model of temporal lobe epilepsy". Hippocampus. 12 (4): 534–42. doi:10.1002/hipo.10031. PMID 12201638. S2CID 24965222.
- ^ Deller, T; Adelmann, G; Nitsch, R; Frotscher, M (December 1996). "The alvear pathway of the rat hippocampus". Cell and Tissue Research. 286 (3): 293–303. doi:10.1007/s004410050699. PMID 8929332. S2CID 36438302.
- ^ Harris, E; Stewart, M (23 March 2001). "Propagation of synchronous epileptiform events from subiculum backward into area CA1 of rat brain slices". Brain Research. 895 (1–2): 41–9. doi:10.1016/s0006-8993(01)02023-6. PMID 11259758. S2CID 23300272.
- ^ Gulyás, AI; Görcs, TJ; Freund, TF (1990). "Innervation of different peptide-containing neurons in the hippocampus by GABAergic septal afferents". Neuroscience. 37 (1): 31–44. doi:10.1016/0306-4522(90)90189-b. PMID 1978740. S2CID 24486668.
- ^ Leão, RN; Mikulovic, S; Leão, KE; Munguba, H; Gezelius, H; Enjin, A; Patra, K; Eriksson, A; Loew, LM; Tort, AB; Kullander, K (November 2012). "OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons". Nature Neuroscience. 15 (11): 1524–30. doi:10.1038/nn.3235. PMC 3483451. PMID 23042082.
- ^ Scharfman, HE (25 June 1993). "Activation of dentate hilar neurons by stimulation of the fimbria in rat hippocampal slices". Neuroscience Letters. 156 (1–2): 61–6. doi:10.1016/0304-3940(93)90440-v. PMC 3281807. PMID 8105429.
- ^ Yang, Sunggu; Yang, Sungchil; Moreira, Thais; Hoffman, Gloria; Carlson, Greg C.; Bender, Kevin J.; Alger, Bradley E.; Tang, Cha-Min (2014-09-02). "Interlamellar CA1 network in the hippocampus". Proceedings of the National Academy of Sciences. 111 (35): 12919–12924. Bibcode:2014PNAS..11112919Y. doi:10.1073/pnas.1405468111. ISSN 0027-8424. PMC 4156755. PMID 25139992.
- ^ Hunsaker, Michael R.; Kesner, Raymond P. (2013-01-01). "The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory". Neuroscience & Biobehavioral Reviews. 37 (1): 36–58. doi:10.1016/j.neubiorev.2012.09.014. PMID 23043857. S2CID 22493885.
- ^ Swanson, LW; Wyss, JM; Cowan, WM (15 October 1978). "An autoradiographic study of the organization of intrahippocampal association pathways in the rat". The Journal of Comparative Neurology. 181 (4): 681–715. doi:10.1002/cne.901810402. PMID 690280. S2CID 30954240.
- ^ Amaral, DG; Witter, MP (1989). "The three-dimensional organization of the hippocampal formation: a review of anatomical data". Neuroscience. 31 (3): 571–91. doi:10.1016/0306-4522(89)90424-7. PMID 2687721. S2CID 28430607.
- ^ Kohara, K; Pignatelli, M; Rivest, AJ; Jung, HY; Kitamura, T; Suh, J; Frank, D; Kajikawa, K; Mise, N; Obata, Y; Wickersham, IR; Tonegawa, S (February 2014). "Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits" (PDF). Nature Neuroscience. 17 (2): 269–79. doi:10.1038/nn.3614. PMC 4004172. PMID 24336151.
- ^ Blackstad, TW (October 1956). "Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination". The Journal of Comparative Neurology. 105 (3): 417–537. doi:10.1002/cne.901050305. PMID 13385382. S2CID 41672064.
- ^ Fricke, R; Cowan, WM (15 September 1978). "An autoradiographic study of the commissural and ipsilateral hippocampo-dentate projections in the adult rat". The Journal of Comparative Neurology. 181 (2): 253–69. doi:10.1002/cne.901810204. PMID 567658. S2CID 46320248.
- ^ Amaral, DG; Scharfman, HE; Lavenex, P (2007). "The dentate gyrus: Fundamental neuroanatomical organization (Dentate gyrus for dummies)". The Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications. Progress in Brain Research. Vol. 163. pp. 3–22. doi:10.1016/S0079-6123(07)63001-5. ISBN 9780444530158. PMC 2492885. PMID 17765709.
- ^ Ribak, CE; Seress, L; Peterson, GM; Seroogy, KB; Fallon, JH; Schmued, LC (December 1986). "A GABAergic inhibitory component within the hippocampal commissural pathway". The Journal of Neuroscience. 6 (12): 3492–8. doi:10.1523/JNEUROSCI.06-12-03492.1986. PMC 6568657. PMID 2432200.
- ^ Sik, Attila; Penttonen, Markku; Buzsáki, György (March 1997). "Interneurons in the Hippocampal Dentate Gyrus: an In Vivo intracellular Study". European Journal of Neuroscience. 9 (3): 573–588. doi:10.1111/j.1460-9568.1997.tb01634.x. PMID 9104599. S2CID 25960013.
- ^ a b Andersen, Per; et al. (2007). The Hippocampus Book. Oxford University press.
- ^ Blackstad, TW (1956). "Commissural connections of the hippocampal region in the rat, with special reference to their mode of termi- nation". J Comp Neurol. 105 (3): 417–537. doi:10.1002/cne.901050305. PMID 13385382. S2CID 41672064.
- ^ Amaral, DG (1978). "A Golgi study of cell types in the hilar region of the hippocampus in the rat". J Comp Neurol. 182 (5): 851–914. doi:10.1002/cne.901820508. PMID 730852. S2CID 44257239.
- ^ "Hippocampome". hippocampome.org.
External links
[edit]- Schematic Diagram of a Hippocampal Brain Slice
- Hippocampus at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Stained brain slice images which include the "hippocampus" at the BrainMaps project
- Hippocampus anatomy and connectivity
