Aldonic acid
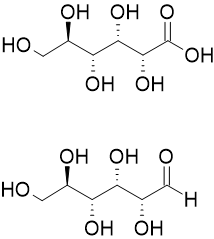
Aldonic acids are sugar acids with the general chemical formula, HO2C(CHOH)nCH2OH. They are obtained by oxidizing the aldehyde (-CHO group) of an aldose to form a carboxylic acid (-COOH group).[1] Aldonic acids are generally found in their ring form. However, these rings do not have a chiral carbon at the terminal end bearing the aldehyde, and they cannot form R−O−R′ linkages between different molecules.[2]
The nomenclature of aldonic acids and their lactones is based on replacing the suffix "-ose" with "onic acid" or "onolactone". Hence, D-glucose is oxidized to D-gluconic acid and D-gluconolactone.[3]
Inventory
[edit]Sugar acids are white, water-soluble solids. They tend to dehydrate to the lactone derivative, often before they can be melted. All are chiral and, at least in nature, enantiopure.
| Compoud | RN | melting point (C) | parent sugar |
|---|---|---|---|
| L-Threonic acid | 7306-96-9 | 143 | threose |
| D-Ribonic acid | 642-98-8 | 143 | ribose |
| D-Xylonic acid | 526-91-0 | - | xylose |
| D-Arabinonic acid | 488-30-2 | 135-136 | arabinose |
| D-Lyxonic acid | 526-92-1 | - | lyxose |
| Gluconic acid | 526-95-4 | 131 | glucose |
| D-Gulonic acid | 526-97-6 | - | gulose |
| D-Galactonic acid | 576-36-3 | - | galactose |
| D-Mannonic acid[4] | 642-99-9 | 74-76 | mannose |
| L-Idonic acid | 1114-17-6 | - | idose |
Synthesis
[edit]Oxidation by bromine and water
[edit]Aldonic acids are most commonly prepared by the oxidation of the sugar with bromine and water under neutral pH.[5]

Strecker reaction
[edit]Alternatively, they arise by homologation of an aldose using the Strecker reaction.[6] Cyanide in ammonia reacts with an aldose to produce an intermediate, which is then reacted with a hydronium ion to form an aldonic acid.
Oxidation by Benedict's and Fehling's reagents
[edit]Aldonic acids are the products of the oxidation of aldoses by Benedict's or Fehling's reagents.[7] Copper ions react with an aldose to form a red precipitate, Cu2O.

Natural synthesis
[edit]Anaerobic bacteria can also perform dehydrogenation to produce aldonic acids.[8] This is done by synthesizing enzymes that are able to selectively oxidize aldoses to their corresponding aldonic acid.
Applications
[edit]In commercial settings, glucose, galactose, or arabinose are commonly oxidized to obtain aldonic acids.[8] These products can then be used as the building blocks for preservatives, buffering agents, and other chemicals.[8] As such, the use of aldonic acids for chemical applications is of growing interest to various industries.
Aldonic acids can be used as the natural starting materials to synthetic products[9] including polyesters and polyurethane.[10] The incorporation of these organic sugars into synthetic materials allow for a more renewable alternative to oil-based polymer synthesis,[10] and increased structural durability within polymer chains.[11]
Properties
[edit]Aldonic acids are typically used in industrial applications for their ability to degrade naturally in the environment.[10] This can be attributed to their affinity with water, as the polar bonds within the carboxylic acid group of aldonic acids allow them to interact with aquatic systems.[12]
The structural diversity of aldonic acids also allow for various properties. Their ring formation creates an added layer of rigidity when integrated with other materials.[11]
See also
[edit]References
[edit]- ^ Gold, Victor, ed. (2019). The IUPAC Compendium of Chemical Terminology: The Gold Book (4 ed.). Research Triangle Park, NC: International Union of Pure and Applied Chemistry (IUPAC). doi:10.1351/goldbook.a00212.
- ^ Sartori, Suélen Karine; Diaz, Marisa Alves Nogueira; Diaz-Muñoz, Gaspar (2021-03-26). "Lactones: Classification, synthesis, biological activities, and industrial applications". Tetrahedron. 84: 132001. doi:10.1016/j.tet.2021.132001. ISSN 0040-4020.
- ^ Robyt, John F. (1998). Essentials of carbohydrate chemistry. Springer advanced texts in chemistry. New York: Springer. ISBN 978-0-387-94951-2.
- ^ Pezzotti, F.; Therisod, M. (2006). "Enzymatic synthesis of aldonic acids". Carbohydrate Research. 341 (13): 2290–2292. doi:10.1016/j.carres.2006.05.023. PMID 16806132.
- ^ Isbell, Horace S. (March 1962). "Oxidation of aldoses with bromine". Journal of Research of the National Bureau of Standards, Section A. 66A (3): 233–239. doi:10.6028/jres.066a.023. ISSN 0022-4332. PMC 5310681.
- ^ "d-gulonic-y-lactone". Organic Syntheses. 36: 38. 1956. doi:10.15227/orgsyn.036.0038. ISSN 0078-6209.
- ^ Simoni, Robert D.; Hill, Robert L.; Vaughan, Martha (April 2002). "Benedict's Solution, a Reagent for Measuring Reducing Sugars: the Clinical Chemistry of Stanley R. Benedict". Journal of Biological Chemistry. 277 (16): e5–e6. doi:10.1016/s0021-9258(19)61050-1. ISSN 0021-9258.
- ^ a b c Wieschalka, Stefan; Blombach, Bastian; Bott, Michael; Eikmanns, Bernhard J. (March 2013). "Bio-based production of organic acids with C orynebacterium glutamicum". Microbial Biotechnology. 6 (2): 87–102. doi:10.1111/1751-7915.12013. ISSN 1751-7915. PMC 3917452. PMID 23199277.
- ^ Mehtiö, Tuomas; Toivari, Mervi; Wiebe, Marilyn G.; Harlin, Ali; Penttilä, Merja; Koivula, Anu (2016-09-02). "Production and applications of carbohydrate-derived sugar acids as generic biobased chemicals". Critical Reviews in Biotechnology. 36 (5): 904–916. doi:10.3109/07388551.2015.1060189. ISSN 0738-8551. PMID 26177333.
- ^ a b c Galbis, Juan A.; García-Martín, M. de Gracia; de Paz, M. Violante; Galbis, Elsa (2016-02-10). "Synthetic Polymers from Sugar-Based Monomers". Chemical Reviews. 116 (3): 1600–1636. doi:10.1021/acs.chemrev.5b00242. hdl:11441/154263. ISSN 0009-2665. PMID 26291239.
- ^ a b Gregory, Georgina L.; López-Vidal, Eva M.; Buchard, Antoine (2017-02-14). "Polymers from sugars: cyclic monomer synthesis, ring-opening polymerisation, material properties and applications". Chemical Communications. 53 (14): 2198–2217. doi:10.1039/C6CC09578J. ISSN 1364-548X. PMID 28127607.
- ^ Morzyk-Ociepa, Barbara; Michalska, Danuta; Pietraszko, Adam (January 2004). "Structures and vibrational spectra of indole carboxylic acids. Part I. Indole-2-carboxylic acid". Journal of Molecular Structure. 688 (1–3): 79–86. Bibcode:2004JMoSt.688...79M. doi:10.1016/j.molstruc.2003.09.027. ISSN 0022-2860.
