7-Aminoactinomycin D

| |
| Names | |
|---|---|
| Other names
7-Amino-actinomycin D
| |
| Identifiers | |
3D model (JSmol)
|
|
| 5915844 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.163.188 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C62H87N13O16 | |
| Molar mass | 1270.43 g/mol |
| Appearance | Red to dark purple powder |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H300, H310, H315, H319, H330, H335, H350, H360 | |
| P201, P202, P260, P261, P262, P264, P270, P271, P280, P281, P284, P301+P310, P302+P350, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P312, P320, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
7-Aminoactinomycin D (7-AAD) is a fluorescent chemical compound with a strong affinity for DNA. It is used as a fluorescent marker for DNA in fluorescence microscopy and flow cytometry. It intercalates in double-stranded DNA, with a high affinity for GC-rich regions,[2] making it useful for chromosome banding studies.[3]
Applications
[edit]With an absorption maximum at 546 nm, 7-AAD is efficiently excited using a 543 nm helium–neon laser; it can also be excited with somewhat lower efficiency using a 488 nm or 514 nm argon laser lines. Its emission has a very large Stokes shift with a maximum in the deep red: 647 nm. 7-AAD is therefore compatible with most blue and green fluorophores – and even many red fluorophores – in multicolour applications.
7-AAD does not readily pass through intact cell membranes; if it is to be used as a stain for imaging DNA fluorescence, the cell membrane must be permeabilized or disrupted. This method can be used in combination with formaldehyde fixation of samples.
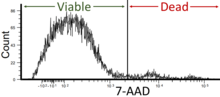
7-AAD is also used as a cell viability stain. Cells with compromised membranes will stain with 7-AAD, while live cells with intact cell membranes will remain dark. Viability of the cells in flow cytometry should be around 95% but not less than 90%.[4]
Actinomycin D
[edit]The related compound actinomycin D is nonfluorescent, but binds DNA in the same way as 7-AAD. Its absorbance changes when bound to DNA, and it can be used as a stain in conventional transmission microscopy.
References
[edit]- ^ 7-Aminoactinomycin D at Interchim
- ^ Liu X; Chen H; Patel D (1991). "Solution structure of actinomycin-DNA complexes: drug intercalation at isolated G-C sites". J Biomol NMR. 1 (4): 323–47. doi:10.1007/BF02192858. PMID 1841703. S2CID 40569430.
- ^ Latt S (1977). "Fluorescent probes of chromosome structure and replication". Can J Genet Cytol. 19 (4): 603–23. doi:10.1139/g77-065. PMID 76502.
- ^ "Flow cytometry (FACS) staining protocol (Cell surface staining)". Yale School of Medicine - Yale Flow Cytometry. Retrieved 2023-10-17.
Gallery
[edit]-
Absorptions
External links
[edit]- Structure from Invitrogen
- MSDS Archived 2014-08-06 at the Wayback Machine

