5α-Reductase
| 3-Oxo-5α-steroid 4-dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC no. | 1.3.1.22 | ||||||||
| CAS no. | 9036-43-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Steroid-5α-reductase 1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | SRD5A1 | ||||||
| NCBI gene | 6715 | ||||||
| HGNC | 11284 | ||||||
| OMIM | 184753 | ||||||
| RefSeq | NM_001047 | ||||||
| UniProt | P18405 | ||||||
| Other data | |||||||
| EC number | 1.3.1.22 | ||||||
| Locus | Chr. 5 p15 | ||||||
| |||||||
| Steroid-5α-reductase 2 | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Symbol | SRD5A2 | ||||||
| NCBI gene | 6716 | ||||||
| HGNC | 11285 | ||||||
| OMIM | 607306 | ||||||
| RefSeq | NM_000348 | ||||||
| UniProt | P31213 | ||||||
| Other data | |||||||
| EC number | 1.3.1.22 | ||||||
| Locus | Chr. 2 p23 | ||||||
| |||||||

5α-Reductases, also known as 3-oxo-5α-steroid 4-dehydrogenases, are enzymes involved in steroid metabolism. They participate in three metabolic pathways: bile acid biosynthesis, androgen and estrogen metabolism. There are three isozymes of 5α-reductase encoded by the genes SRD5A1, SRD5A2, and SRD5A3.
5α-Reductases catalyze the following generalized chemical reaction:
- a 3-oxo-5α-steroid + acceptor ⇌ a 3-oxo-Δ4-steroid + reduced acceptor
Where a 3-oxo-5α-steroid and acceptor are substrates, and a corresponding 3-oxo-Δ4-steroid and the reduced acceptor are products. An instance of this generalized reaction that 5α-reductase type 2 catalyzes is:
- dihydrotestosterone + NADP+ testosterone + NADPH + H+
where dihydrotestosterone is the 3-oxo-5α-steroid, NADP+ is the acceptor and testosterone is the 3-oxo-Δ4-steroid and NADPH the reduced acceptor.
Production and activity
[edit]The enzyme is produced in many tissues in both males and females, in the reproductive tract, testes and ovaries,[1] skin, seminal vesicles, prostate, epididymis and many organs,[2] including the nervous system.[3][4] There are three isoenzymes of 5α-reductase: steroid 5α-reductase 1, 2, and 3 (SRD5A1, SRD5A2 and SRD5A3).[2][5][6][7]
5α-Reductases act on 3-oxo (3-keto), Δ4,5 C19/C21 steroids as its substrates; "3-keto" refers to the double bond of the third carbon to oxygen. Carbons 4 and 5 also have a double bond, represented by 'Δ4,5'. The reaction involves a stereospecific and permanent break of the Δ4,5 with the help of NADPH as a cofactor. A hydride anion (H−) is also placed on the α face at the fifth carbon, and a proton on the β face at carbon 4.[8]
Distribution with age
[edit]5α-R1 is expressed in fetal scalp and nongenital skin of the back, anywhere from 5 to 50 times less than in the adult. 5α-R2 is expressed in fetal prostates similar to adults. 5α-R1 is expressed mainly in the epithelium and 5α-R2 the stroma of the fetal prostate. Scientists looked for 5α-R2 expression in fetal liver, adrenal, testis, ovary, brain, scalp, chest, and genital skin, using immunoblotting, and were only able to find it in genital skin.[8]
After birth, the 5α-R1 is expressed in more locations, including the liver, skin, scalp and prostate. 5α-R2 is expressed in prostate, seminal vesicles, epididymis, liver, and to a lesser extent the scalp and skin. Hepatic expression of both 5α-R1 and 2 is immediate, but disappears in the skin and scalp at month 18. Then, at puberty, only 5α-R2 is reexpressed in the skin and scalp.
5α-R1 and 5α-R2 appear to be expressed in the prostate in male fetuses and throughout postnatal life. 5α-R1 and 5α-R2 are also expressed, although to different degrees in liver, genital and nongenital skin, prostate, epididymis, seminal vesicle, testis, ovary, uterus, kidney, exocrine pancreas, and the brain.[3][8]
In adulthood, 5α-R1-3 [clarification needed] is ubiquitously expressed.
Substrates
[edit]Specific substrates include testosterone, progesterone, androstenedione,[9] epitestosterone, cortisol, aldosterone, and deoxycorticosterone. Outside of dihydrotestosterone, much of the physiological role of 5α-reduced steroids is unknown.[8] Beyond reducing testosterone to dihydrotestosterone, 5alpha-reductase enzyme isoforms I and II reduce progesterone to dihydroprogesterone (DHP) and deoxycorticosterone to dihydrodeoxycorticosterone (DHDOC). In vitro and animal models suggest subsequent 3alpha-reduction of DHT, DHP and DHDOC lead to steroid metabolites with effects on cerebral function achieved by enhancing GABAergic inhibition. These neuroactive steroid derivatives enhance GABA via allosteric modulation at GABA(A) receptors and have anticonvulsant, antidepressant and anxiolytic effects, and also alter sexual and alcohol related behavior.[10] 5α-dihydrocortisol is present in the aqueous humor of the eye, is synthesized in the lens, and might help make the aqueous humor itself.[11] Allopregnanolone and THDOC are neurosteroids, with the latter having effects on the susceptibility of animals to seizures. In socially isolated mice, 5α-R1 is specifically down-regulated in glutamatergic pyramidal neurons that converge on the amygdala from cortical and hippocampal regions. This down-regulation may account for the appearance of behavioral disorders such as anxiety, aggression, and cognitive dysfunction.[3][4] 5α-dihydroaldosterone is a potent antinatriuretic agent, although different from aldosterone. Its formation in the kidney is enhanced by restriction of dietary salt, suggesting it may help retain sodium as follows:[12]

This is the mechanism of 5α-reductase on testosterone to convert it into DHT (dihydrotestosterone). 5α-Reductase works by using the reducing power of NADPH to perform a hydride shift on the double carbon bond in the ring causing enolate formation and subsequent tautamerization to form DHT.[13]
5α-DHP is a major hormone in circulation of normal cycling and pregnant women.[14]
Testosterone
[edit]5α-Reductase is most known for converting testosterone, the male sex hormone, into the more potent dihydrotestosterone:
The major difference is the Δ4,5 double-bond on the A (leftmost) ring. The other differences between the diagrams are unrelated to structure.
List of conversions
[edit]The following reactions are known to be catalyzed by 5α-reductase:[9]
- Cholestenone → 5α-Cholestanone
- Progesterone → 5α-Dihydroprogesterone
- 3α-Dihydroprogesterone → Allopregnanolone
- 3β-Dihydroprogesterone → Isopregnanolone
- Deoxycorticosterone → 5α-Dihydrodeoxycorticosterone
- Corticosterone → 5α-Dihydrocorticosterone
- Aldosterone → 5α-Dihydroaldosterone
- Androstenedione → 5α-Androstanedione
- Testosterone → 5α-Dihydrotestosterone
- Nandrolone → 5α-Dihydronandrolone
Structure
[edit]
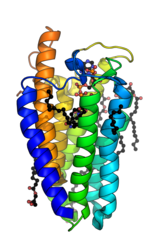
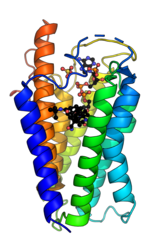
5α-Reductase is a membrane bound enzyme that catalyzes the NADPH dependent reduction of double bonds in steroid substrates to increase potency.[15] The crystal structure of a homolog of 5α-reductase isoenzymes 1 and 2 has been found in Proteobacteria (proteobacteria 5α-reductase). This exists as a monomer with a seven alpha-helix transmembrane structure housing a hydrophobic pocket that holds cofactor NADPH and monoolein which occupies the steroid substrate binding pocket.[16] In insect cells monoolein is not found, but is subbed out for other androgens and inhibitors.[17] The integral seven transmembrane topology is likely conserved across species, with the N terminus in the endoplasmic reticulum lumen and the C terminus facing the cytosol. High conformational dynamics of the cytosolic region likely regulate NADPH/NADP+ exchange.[17] Sequence conservation across known crystal structures has corroborated high conservation in enzyme structure.[16]
Inhibition
[edit]The mechanism of 5α reductase inhibition is complex, but involves the binding of NADPH to the enzyme followed by the substrate. 5α-Reductase inhibitor drugs are used in benign prostatic hyperplasia, prostate cancer, pattern hair loss (androgenetic alopecia), and hormone replacement therapy for transgender women.
Inhibition of the enzyme can be classified into two categories: steroidal, which are irreversible, and nonsteroidal. There are more steroidal inhibitors, with examples including finasteride (MK-906), dutasteride (GG745), 4-MA, turosteride, MK-386, MK-434, and MK-963. Researchers have pursued synthesis of nonsteroidals to inhibit 5α-reductase due to the undesired side effects of steroidals. The most potent and selective inhibitors of 5α-R1 are found in this class, and include benzoquinolones, nonsteroidal aryl acids, butanoic acid derivatives, and more recognizably, polyunsaturated fatty acids (especially linolenic acid), zinc, and green tea.[8] Riboflavin was also identified as a 5α-reductase inhibitor .[18]
Additionally, it has been claimed that alfatradiol works through this mechanism of activity (5α-reductase), as well as the Ganoderic acids in lingzhi mushroom, and the Saw Palmetto.
Inhibition of 5α-reductase results in decreased conversion of testosterone to DHT, leading to increased testosterone and estradiol. Other enzymes compensate to a degree for the absent conversion, specifically with local expression at the skin of reductive 17β-hydroxysteroid dehydrogenase, oxidative 3α-hydroxysteroid dehydrogenase, and 3β-hydroxysteroid dehydrogenase enzymes.[19]
Gynecomastia, erectile dysfunction, impaired cognitive function, fatigue, hypoglycemia, impaired liver function, constipation, and depression, are only a few of the possible side-effects of 5α-reductase inhibition. Long term side effects, that continued even after discontinuation of the drug have been reported.[20]
Finasteride
[edit]Finasteride inhibits two 5α-reductase isoenzymes (II and III), while dutasteride inhibits all three.[2] Finasteride potently inhibits 5α-R2 at a mean inhibitory concentration IC50 of 69 nM, but is less effective with 5α-R1 with an IC50 of 360 nM.[21] Finasteride decreases mean serum level of DHT by 71% after 6 months,[22] and was shown in vitro to inhibit 5α-R3 at a similar potency to 5α-R2 in transfected cell lines.[2]
Dutasteride
[edit]Dutasteride inhibits 5α-reductase isoenzymes type 1 and 2 better than finasteride, leading to a more complete reduction in DHT at 24 weeks (94.7% versus 70.8%).[23] It also reduces intraprostatic DHT 97% in men with prostate cancer at 5 mg/day over three months.[24] A second study with 3.5 mg/day for 4 months decreased intraprostatic DHT even further by 99%.[25] The suppression of DHT in vivo, and the report that dutasteride inhibits 5α-R3 in vitro[26] suggest that dutasteride may be a triple 5α reductase inhibitor.[8]

Congenital deficiencies
[edit]5α-Reductase 1
[edit]5α-Reductase type 1 inactivated male mice have reduced bone mass and forelimb muscle grip strength, which has been proposed to be due to lack of 5α-reductase type 1 expression in bone and muscle.[29] In 5 alpha reductase type 2 deficient males, the type 1 isoenzyme is thought to be responsible for their virilization at puberty.[6]
5α-Reductase 2
[edit]Impaired 5α-reductase 2 activity can result from mutations in the underlying SRD5A2 gene. The condition, known as 5α-reductase 2 deficiency, has a range of presentations as atypical appearances of the external genitalia in males. This is because 5α-reductase 2 catalyzes the transformation of testosterone to the potent androgen dihydrotestosterone, which is required for the proper masculinization of male genitalia.[30]
5α-Reductase 3
[edit]When small interfering RNA is used to knock down the expression of 5α-R3 isozyme in cell lines, there is decreased cell growth, viability, and a decrease in DHT/T ratios.[31] It has also shown the ability to reduce testosterone, androstenedione, and progesterone in androgen stimulated prostate cell lines by adenovirus vectors.[8]
Congenital deficiency of 5α-R3 at the gene SRD53A has been linked to a rare, autosomal recessive condition in which patients are born with severe intellectual dysfunction and cerebellar and ocular defects. The presumed deficiency is reduction of the terminal bond of polyprenol to dolichol, an important step in N-glycosylation of proteins, which in turn is important for proper folding of asparagine residues on nascent protein in the endoplasmic reticulum.[32]
Nervous system
[edit]Affective disorders
[edit]Isolation rearing has been shown to lower protein expression of 5α-reductase isoenzymes 1 and 2 in cortical and subcortical brain regions of rat models. However, the amount of 5α-reduced metabolite remained unaffected. This means isolation rearing likely leads to changes in the expression and activity of 5α-reductase in the brain, leading to dysregulation of dopamine neurotransmission, resulting in early chronic stress[33] Treatment with finasteride, a 5α-reductase inhibitor, has been shown to mimic the effects of SSRI's causing sexual dysfunction.[34] Research has shown that 5α-reductase is the rate-limiting enzyme in neurosteroid synthesis, specifically in the conversion of progesterone to allopregnanolone,[35] low levels of allopregnanolone has been tied to depression, anxiety and schizophrenia. Sleep deprivation can enhance 5α-reductase expression and activity in the prefrontal cortex, leading to mania-related symptoms in rats.[35] It is also contested whether the use of 5α-reductase inhibitors is associated with suicidal ideation and depression in patient populations who use them for benign prostatic hyperplasia.[36][37] These symptoms have been found during active use of inhibitors and in immediate followup.[36] However, it is unknown if these symptoms arise naturally from benign prostatic hyperplasia.[37]
Hypothalamic–pituitary–adrenal axis dysfunction
[edit]An alternative mechanism of cortisol regulation is regulated via 5α-reductase which catalyzes an A-ring reduction of cortisol, metabolizing the compound.[38] Type 1 and 2 of 5α-reductase are the principal enzymes involved in cortisol clearance through the liver.[39] Excess cortisol has been tied to non-alcoholic fatty liver disease (NAFLD), but in-vitro studies have found that an over expression of 5α-reductase type 2 can suppress lipogenesis.[40] The key role of 5α-reductase in cortisol breakdown and fat buildup has elucidated some of the side effects of 5α-reductase inhibitors. In randomized studies on human volunteers it was found that 5α-reductase inhibition through the use of dutasteride and finasteride can lead to hepatic lipid accumulation in men.[41] In critical illness, overstimulation of cortisol as part of a stress response can lead to decreased clearance of cortisol through the liver via 5α-reductase and kidneys via 11β-hydroxysteroid dehydrogenase type 2,[39] longterm elevation of cortisol can lead to Cushing's syndrome.
Nomenclature
[edit]This enzyme belongs to the family of oxidoreductases, to be specific, those acting on the CH-CH group of donor with other acceptors. The systematic name of this enzyme class is 3-oxo-5α-steroid:acceptor Δ4-oxidoreductase. Other names in common use include:
- 5α-Reductase
- 3-Oxosteroid Δ4-dehydrogenase
- 3-Oxo-5α-steroid Δ4-dehydrogenase
- Steroid Δ4-5α-reductase
- Δ4-3-Keto steroid 5α-reductase
- Δ4-3-Oxo steroid reductase
- Δ4-3-Ketosteroid-5α-oxidoreductase
- Δ4-3-Oxosteroid-5α-reductase
- 3-Keto-Δ4-steroid-5α-reductase
- Testosterone 5α-reductase
- 4-Ene-3-ketosteroid-5α-oxidoreductase
- Δ4-5α-Dehydrogenase
- 3-Oxo-5α-steroid:(acceptor) Δ4-oxidoreductase
See also
[edit]- Steroidogenic enzyme
- Acne vulgaris
- Cholestenone 5α-reductase
- Hirsutism
- Lower urinary tract symptoms
- Polycystic ovarian syndrome
- List of steroid metabolism modulators
References
[edit]- ^ Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E (October 2008). "Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice". Neurochemical Research. 33 (10): 1990–2007. doi:10.1007/s11064-008-9718-5. PMID 18473173. S2CID 19338424.
- ^ a b c d Yamana K, Labrie F, Luu-The V (August 2010). "Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride". Hormone Molecular Biology and Clinical Investigation. 2 (3): 293–9. doi:10.1515/hmbci.2010.035. PMID 25961201. S2CID 28841145.
- ^ a b c Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, et al. (September 2006). "Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis". Proceedings of the National Academy of Sciences of the United States of America. 103 (39): 14602–7. Bibcode:2006PNAS..10314602A. doi:10.1073/pnas.0606544103. PMC 1600006. PMID 16984997.
- ^ a b Agís-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A (November 2007). "Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice". Proceedings of the National Academy of Sciences of the United States of America. 104 (47): 18736–41. Bibcode:2007PNAS..10418736A. doi:10.1073/pnas.0709419104. PMC 2141846. PMID 18003893.
- ^ Killian J, Pratis K, Clifton RJ, Stanton PG, Robertson DM, O'Donnell L (May 2003). "5alpha-reductase isoenzymes 1 and 2 in the rat testis during postnatal development". Biology of Reproduction. 68 (5): 1711–8. doi:10.1095/biolreprod.102.009142. PMID 12606426.
- ^ a b Thiele S, Hoppe U, Holterhus PM, Hiort O (June 2005). "Isoenzyme type 1 of 5alpha-reductase is abundantly transcribed in normal human genital skin fibroblasts and may play an important role in masculinization of 5alpha-reductase type 2 deficient males". European Journal of Endocrinology. 152 (6): 875–80. doi:10.1530/eje.1.01927. PMID 15941927.
- ^ Godoy A, Kawinski E, Li Y, Oka D, Alexiev B, Azzouni F, et al. (July 2011). "5α-reductase type 3 expression in human benign and malignant tissues: a comparative analysis during prostate cancer progression". The Prostate. 71 (10): 1033–46. doi:10.1002/pros.21318. PMC 4295561. PMID 21557268.
- ^ a b c d e f g Azzouni F, Godoy A, Li Y, Mohler J (2012). "The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases". Advances in Urology. 2012: 530121. doi:10.1155/2012/530121. PMC 3253436. PMID 22235201.
- ^ a b Paba S, Frau R, Godar SC, Devoto P, Marrosu F, Bortolato M (2011). "Steroid 5α-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders". Current Pharmaceutical Design. 17 (2): 151–67. doi:10.2174/138161211795049589 (inactive 1 November 2024). PMID 21361868.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, et al. (2006). "A new look at the 5alpha-reductase inhibitor finasteride". CNS Drug Reviews. 12 (1): 53–76. doi:10.1111/j.1527-3458.2006.00053.x. PMC 6741762. PMID 16834758.
- ^ Weinstein BI, Kandalaft N, Ritch R, Camras CB, Morris DJ, Latif SA, et al. (June 1991). "5 alpha-dihydrocortisol in human aqueous humor and metabolism of cortisol by human lenses in vitro". Investigative Ophthalmology & Visual Science. 32 (7): 2130–5. PMID 2055703.
- ^ Kenyon CJ, Brem AS, McDermott MJ, Deconti GA, Latif SA, Morris DJ (May 1983). "Antinatriuretic and kaliuretic activities of the reduced derivatives of aldosterone". Endocrinology. 112 (5): 1852–6. doi:10.1210/endo-112-5-1852. PMID 6403339.
- ^ Ahmed S, Denison S (September 1998). "Mechanism based representation of the active site of 5 alpha-reductase (5AR)". Bioorganic & Medicinal Chemistry Letters. 8 (18): 2615–70. doi:10.1016/S0960-894X(98)00463-6. PMID 9873591.
- ^ Milewich L, Gomez-Sanchez C, Crowley G, Porter JC, Madden JD, MacDonald PC (October 1977). "Progesterone and 5alpha-pregnane-3,20-dione in peripheral blood of normal young women: Daily measurements throughout the menstrual cycle". The Journal of Clinical Endocrinology and Metabolism. 45 (4): 617–22. doi:10.1210/jcem-45-4-617. PMID 914969.
- ^ Wilson JD (8 February 2002). "The role of 5alpha-reduction in steroid hormone physiology". Reproduction, Fertility, and Development. 13 (7–8): 673–8. doi:10.1071/rd01074. PMID 11999320. Archived from the original on 26 May 2021. Retrieved 6 March 2021.
- ^ a b Han Y, Zhuang Q, Sun B, Lv W, Wang S, Xiao Q, et al. (January 2021). "Crystal structure of steroid reductase SRD5A reveals conserved steroid reduction mechanism". Nature Communications. 12 (1): 449. Bibcode:2021NatCo..12..449H. doi:10.1038/s41467-020-20675-2. PMC 7815742. PMID 33469028.
- ^ a b Xiao Q, Wang L, Supekar S, Shen T, Liu H, Ye F, et al. (October 2020). "Structure of human steroid 5α-reductase 2 with the anti-androgen drug finasteride". Nature Communications. 11 (1): 5430. Bibcode:2020NatCo..11.5430X. doi:10.1038/s41467-020-19249-z. PMC 7591894. PMID 33110062.
- ^ Nakayama O, Yagi M, Kiyoto S, Okuhara M, Kohsaka M (December 1990). "Riboflavin, a testosterone 5 alpha-reductase inhibitor". The Journal of Antibiotics. 43 (12): 1615–6. doi:10.7164/antibiotics.43.1615. PMID 2276981.
- ^ Andersson S (2001). "Steroidogenic enzymes in skin". European Journal of Dermatology. 11 (4): 293–5. PMID 11399532.
- ^ Irwig MS, Kolukula S (June 2011). "Persistent sexual side effects of finasteride for male pattern hair loss". The Journal of Sexual Medicine. 8 (6): 1747–53. doi:10.1111/j.1743-6109.2011.02255.x. PMID 21418145.
- ^ Tian G, Stuart JD, Moss ML, Domanico PL, Bramson HN, Patel IR, et al. (March 1994). "17 beta-(N-tert-butylcarbamoyl)-4-aza-5 alpha-androstan-1-en-3-one is an active site-directed slow time-dependent inhibitor of human steroid 5 alpha-reductase 1". Biochemistry. 33 (8): 2291–6. doi:10.1021/bi00174a041. PMID 8117686.
- ^ McConnell JD, Wilson JD, George FW, Geller J, Pappas F, Stoner E (March 1992). "Finasteride, an inhibitor of 5 alpha-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia". The Journal of Clinical Endocrinology and Metabolism. 74 (3): 505–8. doi:10.1210/jcem.74.3.1371291. PMID 1371291.
- ^ Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S (May 2004). "Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor". The Journal of Clinical Endocrinology and Metabolism. 89 (5): 2179–84. doi:10.1210/jc.2003-030330. PMID 15126539.
- ^ Andriole GL, Humphrey P, Ray P, Gleave ME, Trachtenberg J, Thomas LN, et al. (September 2004). "Effect of the dual 5alpha-reductase inhibitor dutasteride on markers of tumor regression in prostate cancer". The Journal of Urology. 172 (3): 915–9. doi:10.1097/01.ju.0000136430.37245.b9. PMID 15310997.
- ^ Gleave M, Qian J, Andreou C, Pommerville P, Chin J, Casey R, et al. (November 2006). "The effects of the dual 5alpha-reductase inhibitor dutasteride on localized prostate cancer--results from a 4-month pre-radical prostatectomy study". The Prostate. 66 (15): 1674–85. doi:10.1002/pros.20499. PMID 16927304. S2CID 40446842.
- ^ Moss GP (December 1989). "IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). The nomenclature of steroids. Recommendations 1989". European Journal of Biochemistry. 186 (3): 429–58. doi:10.1111/j.1432-1033.1989.tb15228.x. PMID 2606099.
- ^ Drury JE, Di Costanzo L, Penning TM, Christianson DW (July 2009). "Inhibition of human steroid 5beta-reductase (AKR1D1) by finasteride and structure of the enzyme-inhibitor complex". The Journal of Biological Chemistry. 284 (30): 19786–90. doi:10.1074/jbc.c109.016931. PMC 2740403. PMID 19515843.
- ^ Hulin-Curtis SL, Petit D, Figg WD, Hsing AW, Reichardt JK (December 2010). "Finasteride metabolism and pharmacogenetics: new approaches to personalized prevention of prostate cancer". Future Oncology. 6 (12): 1897–913. doi:10.2217/fon.10.149. PMC 6300128. PMID 21142863.
- ^ Windahl SH, Andersson N, Börjesson AE, Swanson C, Svensson J, Movérare-Skrtic S, et al. (2011). Vanacker JM (ed.). "Reduced bone mass and muscle strength in male 5α-reductase type 1 inactivated mice". PLOS ONE. 6 (6): e21402. Bibcode:2011PLoSO...621402W. doi:10.1371/journal.pone.0021402. PMC 3120862. PMID 21731732.
- ^ Sinnecker GH, Hiort O, Dibbelt L, Albers N, Dörr HG, Hauß H, et al. (3 May 1996). "Phenotypic classification of male pseudohermaphroditism due to steroid 5α-reductase 2 deficiency". American Journal of Medical Genetics. 63 (1): 223–230. doi:10.1002/(SICI)1096-8628(19960503)63:1<223::AID-AJMG39>3.0.CO;2-O. PMID 8723114.
- ^ Uemura M, Tamura K, Chung S, Honma S, Okuyama A, Nakamura Y, et al. (January 2008). "Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer". Cancer Science. 99 (1): 81–6. doi:10.1111/j.1349-7006.2007.00656.x. PMC 11158902. PMID 17986282. S2CID 51733620.
- ^ Cantagrel V, Lefeber DJ, Ng BG, Guan Z, Silhavy JL, Bielas SL, et al. (July 2010). "SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder". Cell. 142 (2): 203–17. doi:10.1016/j.cell.2010.06.001. PMC 2940322. PMID 20637498.
- ^ Bortolato M, Devoto P, Roncada P, Frau R, Flore G, Saba P, et al. (June 2011). "Isolation rearing-induced reduction of brain 5α-reductase expression: relevance to dopaminergic impairments". Neuropharmacology. 60 (7–8): 1301–8. doi:10.1016/j.neuropharm.2011.01.013. PMID 21256141. S2CID 20164197.
- ^ Giatti S, Diviccaro S, Panzica G, Melcangi RC (August 2018). "Post-finasteride syndrome and post-SSRI sexual dysfunction: two sides of the same coin?". Endocrine. 61 (2): 180–193. doi:10.1007/s12020-018-1593-5. PMID 29675596. S2CID 4974636.
- ^ a b Frau R, Bini V, Soggiu A, Scheggi S, Pardu A, Fanni S, et al. (October 2017). "The Neurosteroidogenic Enzyme 5α-Reductase Mediates Psychotic-Like Complications of Sleep Deprivation". Neuropsychopharmacology. 42 (11): 2196–2205. doi:10.1038/npp.2017.13. PMC 5603808. PMID 28102229.
- ^ a b Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S (May 2017). "Association of Suicidality and Depression With 5α-Reductase Inhibitors". JAMA Internal Medicine. 177 (5): 683–691. doi:10.1001/jamainternmed.2017.0089. PMC 5818776. PMID 28319231.
- ^ a b Dyson TE, Cantrell MA, Lund BC (October 2020). "Lack of Association between 5α-Reductase Inhibitors and Depression". The Journal of Urology. 204 (4): 793–798. doi:10.1097/JU.0000000000001079. PMID 32294395. S2CID 215794669.
- ^ Petrescu AD, Kain J, Liere V, Heavener T, DeMorrow S (2018). "Hypothalamus-Pituitary-Adrenal Dysfunction in Cholestatic Liver Disease". Frontiers in Endocrinology. 9: 660. doi:10.3389/fendo.2018.00660. PMC 6240761. PMID 30483216.
- ^ a b Boonen E, Vervenne H, Meersseman P, Andrew R, Mortier L, Declercq PE, et al. (April 2013). "Reduced cortisol metabolism during critical illness". The New England Journal of Medicine. 368 (16): 1477–88. doi:10.1056/NEJMoa1214969. PMC 4413428. PMID 23506003.
- ^ Nasiri M, Nikolaou N, Parajes S, Krone NP, Valsamakis G, Mastorakos G, et al. (August 2015). "5α-Reductase Type 2 Regulates Glucocorticoid Action and Metabolic Phenotype in Human Hepatocytes". Endocrinology. 156 (8): 2863–71. doi:10.1210/en.2015-1149. PMC 4511138. PMID 25974403.
- ^ Hazlehurst JM, Oprescu AI, Nikolaou N, Di Guida R, Grinbergs AE, Davies NP, et al. (January 2016). "Dual-5α-Reductase Inhibition Promotes Hepatic Lipid Accumulation in Man". The Journal of Clinical Endocrinology and Metabolism. 101 (1): 103–13. doi:10.1210/jc.2015-2928. PMC 4701851. PMID 26574953.
Further reading
[edit]- Levy HR, Talalay P (August 1959). "Bacterial oxidation of steroids. II. Studies on the enzymatic mechanism of ring A dehydrogenation". The Journal of Biological Chemistry. 234 (8): 2014–21. doi:10.1016/S0021-9258(18)69859-X. PMID 13673006.
External links
[edit]- Testosterone+5-alpha-Reductase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)





