3-Hydroxyacetophenone
Appearance
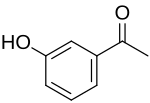
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-(3-Hydroxyphenyl)ethan-1-one | |
| Other names
1-(3-Hydroxyphenyl)ethanone
3-Acetylphenol m-Hydroxyacetophenone 3'-Hydroxyacetophenone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.004.086 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H8O2 | |
| Molar mass | 136.150 g·mol−1 |
| Density | 1.099 g/cm3 |
| Melting point | 96 °C (205 °F; 369 K) |
| Boiling point | 296 °C (565 °F; 569 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
3-Hydroxyacetophenone is a chemical compound. It is a component of castoreum, the exudate from the castor sacs of the mature beaver.[1]
Related compounds
[edit]Humans excrete small amounts of conjugated 2-amino-3-hydroxyacetophenone, a product of tryptophan metabolism, in the urine.[2]
The plant Chrysothamnus viscidiflorus (Asteraceae) contains an m-hydroxyacetophenone named viscidone.[3]
See also
[edit]References
[edit]- ^ Müller-Schwarze, D.; Houlihan, Peter W. (1991). "Pheromonal activity of single castoreum constituents in beaver, Castor canadensis". Journal of Chemical Ecology. 17 (4): 715–34. Bibcode:1991JCEco..17..715M. doi:10.1007/BF00994195. PMID 24258917. S2CID 29937875.
- ^ Dalgliesh, CE (1955). "Excretion of conjugated 2-amino-3-hydroxyacetophenone by man, and its significance in tryptophan metabolism". Biochemical Journal. 61 (2): 334–337. doi:10.1042/bj0610334. PMC 1215790. PMID 13260216.
- ^ Ngo, le-van; Thi, Van Cuong Pham (1981). "An unusual m-hydroxyacetophenone and three new chromanone derivatives from Chrysothamnus viscidiflorus". Phytochemistry. 20 (3): 485. Bibcode:1981PChem..20..485N. doi:10.1016/S0031-9422(00)84171-0.
