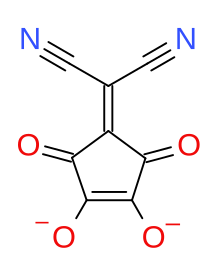
2-(Dicyanomethylene)croconate
Appearance
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-(Dicyanomethylene)-3,5-dioxo-1-cyclopentene-1,2-diolate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8N2O42− | |
| Molar mass | 188.099 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-(Dicyanomethylene)croconate is a divalent anion with chemical formula C
8N
2O2−
4 or ((N≡C−)2C=)(C5O4)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of one oxygen atom by a dicyanomethylene group =C(−C≡N)2.
The anion was synthesized and characterized by A. Fatiadi in 1980, by hydrolysis of croconate violet treated with potassium hydroxide.[1] It gives an orange solution in water.
See also
[edit]- Croconate violet, 1,3-bis(dicyanomethylene)croconate
- Croconate blue, 1,2,3-tris(dicyanomethylene)croconate
- 1,2-Bis(dicyanomethylene)squarate
- 1,3-Bis(dicyanomethylene)squarate
References
[edit]- ^ Alexander J. Fatiadi (1980), "Pseudooxocarbons. Synthesis of 1,2,3-tris(dicyanomethylene)croconate salts. A new bond-delocalized dianion, croconate blue". Journal of Organic Chemistry volume 45, pages 1338–1339. doi:10.1021/jo01295a044
