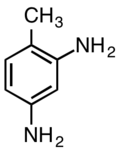
2,4-Diaminotoluene
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Methylbenzene-1,3-diamine | |
| Other names
2,4-Toluenediamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.002.231 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C7H10N2 | |
| Molar mass | 122.171 g·mol−1 |
| Appearance | White solid |
| Density | 1.521 g/cm3 |
| Melting point | 97 to 99 °C (207 to 210 °F; 370 to 372 K) |
| Boiling point | 283 to 285 °C (541 to 545 °F; 556 to 558 K) |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[1] |
REL (Recommended)
|
Ca[1] |
IDLH (Immediate danger)
|
Ca [N.D.][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2,4-Diaminotoluene is an aromatic organic compound with the formula C6H3(NH2)2CH3. It is one isomer of six with this formula. It is a white solid, although commercial samples are often yellow-tan.[2]
Preparation
[edit]It is prepared by hydrogenation of 2,4-dinitrotoluene using a nickel catalyst. Commercial samples often contain up to 20% of the 2,6-isomer.[2]
A laboratory method involves reduction of 2,4-dinitrotoluene with iron powder.[3]
Use
[edit]It is mainly used to manufacture toluene diisocyanate, which is a key raw material in polyurethane chemistry.[4] It is still the starting material used when non-phosgene methods of production of toluene diisocyanate are used.[5][6]
It is also a degradation product of polyurethane materials produced using toluene diisocyanate.

Its reaction with benzenediazonium chloride gives the cationic azo dye Basic Orange 1. Condensation of 2,4-diaminotoluene with acetaldehyde gives the acridine dye called Basic Yellow 9.[7]
Toxicity
[edit]Aromatic amines in general often are classed as toxic and so the toxicity profile of this species has been studied.[8][9][10] It has received more in depth study in the 21st century.[11][12]
References
[edit]- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0620". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Robert A. Smiley "Phenylene- and Toluenediamines" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_405
- ^ Mahood, S. A.; Schaffner, P. V. L. (1931). "2,4-Diaminotoluene". Organic Syntheses. 11: 32. doi:10.15227/orgsyn.011.0032.
- ^ Ji, Lu; Li, Fang; Che, Conghui; Xue, Wei; Yang, Qiusheng; Ding, Xiaoshu; Zhang, Dongsheng; Zhao, Xinqiang; Wang, Yanji (October 2023). "Single-atom Au-modified CeO2 catalyst: Structure and its catalytic performance in 2,4-diaminotoluene methoxycarbonylation reaction". Applied Catalysis A: General. 667: 119459. doi:10.1016/j.apcata.2023.119459. ISSN 0926-860X.
- ^ Sun, Shuai; Liang, Ning; An, Hualiang; Zhao, Xinqiang; Wang, Guirong; Wang, Yanji (2013-06-12). "Kinetics for Dimethyl Toluene-2,4-dicarbamate Synthesis from 2,4-Diaminotoluene, Urea, and Methanol". Industrial & Engineering Chemistry Research. 52 (23): 7684–7689. doi:10.1021/ie4005095. ISSN 0888-5885.
- ^ Juárez, Raquel; Padilla, Ana; Corma, Avelino; García, Hermenegildo (2008-11-05). "Organocatalysts for the Reaction of Dimethyl Carbonate with 2,4-Diaminotoluene". Industrial & Engineering Chemistry Research. 47 (21): 8043–8047. doi:10.1021/ie800126t. ISSN 0888-5885.
- ^ Thomas Gessner and Udo Mayer "Triarylmethane and Diarylmethane Dyes" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim.doi:10.1002/14356007.a27_179
- ^ National Toxicology Program (1979). "Bioassay of 2,4-diaminotoluene for possible carcinogenicity". National Cancer Institute Carcinogenesis Technical Report Series. 162: 1–139. ISSN 0163-7185. PMID 12799700.
- ^ George, E.; Westmoreland, C. (1991). "Evaluation of the in vivo genotoxicity of the structural analogues 2,6-diaminotoluene and 2,4-diaminotoluene using the rat micronucleus test and rat liver UDS assay". Carcinogenesis. 12 (12): 2233–2237. doi:10.1093/carcin/12.12.2233. ISSN 0143-3334.
- ^ Bermudez, Edilberto; Tillery, Deborah; Butterworth, Byron E. (January 1979). "The effect of 2,4‐diaminotoluene and isomers of dinitrotoluene on unscheduled dna synthesis in primary rat hepatocytes". Environmental Mutagenesis. 1 (4): 391–398. doi:10.1002/em.2860010412. ISSN 0192-2521.
- ^ Femina Carolin, C.; Senthil Kumar, P.; Janet Joshiba, G.; Ramamurthy, Racchana; Varjani, Sunita J. (July 2020). "Bioremediation of 2,4-Diaminotoluene in Aqueous Solution Enhanced by Lipopeptide Biosurfactant Production from Bacterial Strains". Journal of Environmental Engineering. 146 (7). doi:10.1061/(ASCE)EE.1943-7870.0001740. ISSN 0733-9372.
- ^ Séverin, Isabelle; Jondeau, Adeline; Dahbi, Laurence; Chagnon, Marie-Christine (2005-09-15). "2,4-Diaminotoluene (2,4-DAT)-induced DNA damage, DNA repair and micronucleus formation in the human hepatoma cell line HepG2". Toxicology. 213 (1): 138–146. doi:10.1016/j.tox.2005.05.021. ISSN 0300-483X.
