From Wikipedia, the free encyclopedia
Chemical compound
Pharmaceutical compound
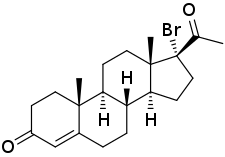
17α-Bromoprogesterone Other names 17α-BP; 17α-Bromopregn-4-ene-3,20-dione Drug class Progestogen ; Progestin
(8R ,9S ,10R ,13S ,14S ,17R )-17-Acetyl-17-bromo-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H -cyclopenta[a ]phenanthren-3-one
CAS Number PubChem CID UNII Formula C 21 H 29 Br O 2 Molar mass −1 3D model (JSmol )
CC(=O)[C@]1(CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CCC4=CC(=O)CC[C@]34C)C)Br
InChI=1S/C21H29BrO2/c1-13(23)21(22)11-8-18-16-5-4-14-12-15(24)6-9-19(14,2)17(16)7-10-20(18,21)3/h12,16-18H,4-11H2,1-3H3/t16-,17+,18+,19+,20+,21+/m1/s1
Key:IENASMFTJRLBJP-CEGNMAFCSA-N
17α-Bromoprogesterone (17α-BP ) is a progestin which was first described in 1957 and was never marketed.[ 1] [ 2] [ 3] [ 4] potent as progesterone in terms of progestogenic activity in animal bioassays .[ 1] parent compound of haloprogesterone (6α-fluoro-17α-bromoprogesterone) and 6α-methyl-17α-bromoprogesterone .[ 5]
^ a b Engel CR, Jahnke H (November 1957). "Steroids and related products. X. 17 alpha-Bromoprogesterone, a new potent gestogen". Canadian Journal of Biochemistry and Physiology . 35 (11): 1047–1055. doi :10.1139/o57-120 . PMID 13479803 . ^ Seeley DH, Wang WY, Salhanick HA (November 1982). "Molecular interactions of progesterone analogues with rabbit uterine cytoplasmic receptor" . The Journal of Biological Chemistry . 257 (22): 13359–13366. doi :10.1016/S0021-9258(18)33456-2 PMID 7142152 . ^ Bohl M, Simon Z, Vlad A, Kaufmann G, Ponsold K (1987). "MTD calculations on quantitative structure-activity relationships of steroids binding to the progesterone receptor" . Zeitschrift für Naturforschung C . 42 (7–8): 935–940. doi :10.1515/znc-1987-7-834 PMID 2961153 . S2CID 22962904 . ^ Simon Z, Bohl M (1992). "Structure-activity Relations in Gestagenic Steroids by the MTD Method. The Case of Hard Molecules and Soft Receptors". Quantitative Structure-Activity Relationships . 11 (1): 23–28. doi :10.1002/qsar.19920110104 . ISSN 0931-8771 . ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies ISBN 978-1-4757-2085-3
PR Tooltip Progesterone receptor
Agonists
Testosterone derivatives: Progestins: 6,6-Difluoronorethisterone 6,6-Difluoronorethisterone acetate 17α-Allyl-19-nortestosterone Allylestrenol Altrenogest Chloroethynylnorgestrel Cingestol Danazol Desogestrel Dienogest Ethinylandrostenediol
Ethisterone Ethynerone Etonogestrel Etynodiol Etynodiol diacetate Gestodene Gestrinone Levonorgestrel Levonorgestrel esters (e.g., levonorgestrel butanoate )Lynestrenol Lynestrenol phenylpropionate Metynodiol Metynodiol diacetate Norelgestromin Norethisterone (norethindrone) Norethisterone esters (e.g., norethisterone acetate , norethisterone enanthate )Noretynodrel Norgesterone Norgestimate Norgestrel Norgestrienone Norvinisterone Oxendolone Quingestanol Quingestanol acetate Tibolone Tigestol Tosagestin ; Anabolic–androgenic steroids: 11β-Methyl-19-nortestosterone 11β-Methyl-19-nortestosterone dodecylcarbonate 19-Nor-5-androstenediol 19-Nor-5-androstenedione 19-Nordehydroepiandrosterone Bolandiol Bolandiol dipropionate Bolandione Dimethisterone Dienedione Dienolone Dimethandrolone Dimethandrolone buciclate Dimethandrolone dodecylcarbonate Dimethandrolone undecanoate Dimethyldienolone Dimethyltrienolone Ethyldienolone Ethylestrenol (ethylnandrol) Methyldienolone Metribolone (R-1881) Methoxydienone (methoxygonadiene) Mibolerone Nandrolone Nandrolone esters (e.g., nandrolone decanoate , nandrolone phenylpropionate )Norethandrolone Normethandrone (methylestrenolone, normethandrolone, normethisterone) RU-2309 Tetrahydrogestrinone Trenbolone (trienolone) Trenbolone esters (e.g., trenbolone acetate , trenbolone enanthate )Trendione Trestolone Trestolone acetate MixedSPRMs Tooltip Selective progesterone receptor modulators ) Antagonists
mPR Tooltip Membrane progesterone receptor PAQR Tooltip Progestin and adipoQ receptor )