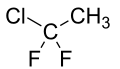
1-Chloro-1,1-difluoroethane
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Chloro-1,1-difluoroethane | |
| Other names
Freon 142b; R-142b; HCFC-142b; Chlorodifluoroethane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.811 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H3ClF2 | |
| Molar mass | 100.49 g·mol−1 |
| Appearance | Colorless gas[1] |
| Melting point | −130.8 °C (−203.4 °F; 142.3 K)[1] |
| Boiling point | −9.6 °C (14.7 °F; 263.5 K)[1] |
| Slight[1] | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Asphyxiant |
| 632 °C (1,170 °F; 905 K)[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1-Chloro-1,1-difluoroethane (HCFC-142b) is a haloalkane with the chemical formula CH3CClF2. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment. It is primarily used as a refrigerant where it is also known as R-142b and by trade names including Freon-142b.[2]
Physiochemical properties
[edit]1-Chloro-1,1-difluoroethane is a highly flammable, colorless gas under most atmospheric conditions. It has a boiling point of -10 °C.[1][3] Its critical temperature is near 137 °C.[4]
Applications
[edit]HCFC-142b is used as a refrigerant, as a blowing agent for foam plastics production, and as feedstock to make polyvinylidene fluoride (PVDF).[5] It was introduced to replace the chlorofluorocarbons (CFCs) that were initially undergoing a phase-out per the Montreal Protocol, but HCFCs still have a significant ozone-depletion ability. As of year 2020, HCFC's are replaced by non ozone depleting HFCs within many applications.[6]
In the United States, the EPA stated that HCFCs could be used in "processes that result in the transformation or destruction of the HCFCs", such as using HCFC-142b as a feedstock to make PVDF. HCFCs could also be used in equipment that was manufactured before January 1, 2010.[7] The point of these new regulations was to phase-out HCFCs in much the same way that CFCs were phased out. HCFC-142b production in non article 5 countries like the United States was banned on January 1, 2020, under the Montreal Protocol.[6]
Production history
[edit]According to the Alternative Fluorocarbons Environmental Acceptability Study (AFEAS), in 2006 global production (excluding India and China who did not report production data) of HCFC-142b was 33,779 metric tons and an increase in production from 2006 to 2007 of 34%.[8]
For the most part, concentrations of HCFCs in the atmosphere match the emission rates that were reported by industries. The exception to this is HCFC-142b which had a higher concentration than the emission rates suggest it should.[9]
Environmental effects
[edit]

The concentration of HCFC-142b in the atmosphere grew to over 20 parts per trillion by year 2010.[10] It has an ozone depletion potential (ODP) of 0.07.[11] This is low compared to the ODP=1 of trichlorofluoromethane (CFC-11, R-11), which also grew about ten times more abundant in the atmosphere by year 1985 (prior to introduction of HCFC-142b and the Montreal Protocol).
HCFC-142b is also a minor but potent greenhouse gas. It has an estimated lifetime of about 17 years and a 100-year global warming potential ranging 2300 to 5000.[12][13] This compares to the GWP=1 of carbon dioxide, which had a much greater atmospheric concentration near 400 parts per million in year 2020.
See also
[edit]References
[edit]- ^ a b c d e f Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ "Safety Data Sheet for 1-Chloro-1,1-difluoroethane" (PDF). Archived from the original (PDF) on 24 October 2018. Retrieved 24 February 2018.
- ^ "Addenda d, j, l, m, and t to ANSI/ASHRAE Standard 34-2004" (PDF). ANSI/ASHRAE Standard 34-2004, Designation and Safety Classification of Refrigerants. Atlanta, GA: American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc. 2007-03-03. ISSN 1041-2336. Archived from the original (PDF) on 2011-10-12. Retrieved 2011-12-18.
- ^ Schoen, J. Andrew, "Listing of Refrigerants" (PDF), Andy's HVAC/R Web Page, archived from the original (PDF) on 2009-03-19, retrieved 2011-12-17
- ^ "Phaseout of Class II Ozone-Depleting Substances". Environmental Protection Agency. 22 July 2015.
- ^ a b "Overview of HCFC Consumption and Available Alternatives For Article 5 Countries" (PDF). ICF International. 2008. Retrieved 2021-02-12.
- ^ U.S. Government Publishing Office Federal Register 2005 November 4, Protection of Stratospheric Ozone: Notice of Data Availability; Information Concerning the Current and Predicted Use of HCFC-22 and HCFC-142b Pages 67172 - 67174 [FR DOC # 05-22036].
- ^ "Production and Sales of Fluorocarbons - AFEAS". Archived from the original on 2015-09-28. Retrieved 2018-02-13.
- ^ "Good news from the stratosphere, sort of: Accumulating HCFCs won't stop ozone-hole mending". Archived from the original on 2016-03-03. Retrieved 2018-02-13.
- ^ a b "HCFC-142b". NOAA Earth System Research Laboratories/Global Monitoring Division. Retrieved 2021-02-12.
- ^ John S. Daniel; Guus J.M. Velders; A.R. Douglass; P.M.D. Forster; D.A. Hauglustaine; I.S.A. Isaksen; L.J.M. Kuijpers; A. McCulloch; T.J. Wallington (2006). "Chapter 8. Halocarbon Scenarios, Ozone Depletion Potentials, and Global Warming Potentials" (PDF). Scientific Assessment of Ozone Depletion: 2006. Geneva, Switzerland: World Meteorological Organization. Retrieved 9 October 2016.
- ^ "Chapter 8". AR5 Climate Change 2013: The Physical Science Basis. p. 731.
- ^ "Refrigerants - Environmental Properties". The Engineering ToolBox. Retrieved 2016-09-12.
