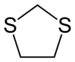
1,3-Dithiolane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,3-Dithiolane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 102455 | |||
| ChEBI | |||
| ChemSpider | |||
| 82036 | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6S2 | |||
| Molar mass | 106.20 g·mol−1 | ||
| Related compounds | |||
Related compounds
|
Ethane-1,2-dithiol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
1,3-Dithiolane is the organosulfur compound with the formula CH2S2C2H4. Also classified as a heterocycle related cyclopentane by replacing two methylene bridges (-CH
2- units) with thioether groups. It is an isomer of 1,2-dithiolane.[1] 1,3-Dithiolanes are compounds where one or more H atoms of the parent 1,3-dithiolane are replaced by other groups. These species are more widely studied.
Synthesis
[edit]A common family of 1,3-dithiolanes have the formula RCHS2C2H4. They are obtained by treating an aldehyde with 1,2-ethanedithiol.[2] Related compounds with the formula R2CS2C2H4 are obtained by condensation of 1,2-ethanedithiol with ketones.[3] The dithiolane protected aldehydes and ketones are amenable to many reactions without perturbing the dithiolane ring.[4]

Dithiolanes can often be reverted, i.e., deprotected,[5] to the parent aldehyde and ketone. A variety of reagents have been developed for that purpose.[6]
Reactions
[edit]1,3-Dithiolanes derived from aldehydes can be deprotonated:
- RCHS2C2H4 + BuLi → RCLiS2C2H4 + BuH
These organolithium compounds degrade with loss of ethylene to give the dthiocarboxylate:
- RCLiS2C2H4 → RCS2Li + C2H4
In contrast, 2-lithio-1,3-dithianes (RCLiS2C3H6) are long-lived.
1,3-Dithiolanes are susceptible to a variety of degradation processes involving organometallic reagents leading to other organosulfur compounds.[2][3]
References
[edit]- ^ Teuber, Lene (1990). "Naturally Occurring 1,2-Dithiolanes and 1,2,3-Trithianes. Chemical and Biological Properties". Sulfur Reports. 9 (4): 257–333. doi:10.1080/01961779008048732.
- ^ a b Ni, Zhi-Jie; Luh, Tien-Yau (1992). "Nickel-Catalyzed Silylolefination of Allylic Dithioacetals: (E,E)-Trimethyl(4-Phenyl-1,3-Butadienyl)Silane". Organic Syntheses. 70: 240. doi:10.15227/orgsyn.070.0240.
- ^ a b Wilson, S. R.; Georgiadis, G. M. (1983). "Mercaptans from Thioketals: Cyclododecyl Mercaptan". Organic Syntheses. 61: 74. doi:10.15227/orgsyn.061.0074.
- ^ Dahnke, Karl R.; Paquette, Leo A. (1993). "2-Methylene-1,3-Dithiolane". Organic Syntheses. 71: 175. doi:10.15227/orgsyn.071.0175.
- ^ Wuts, P. G. M.; Greene, T. W. (2006). Greene's Protective Groups in Organic Synthesis. NY: J. Wiley. doi:10.1002/0470053488. ISBN 9780470053485. S2CID 83393227.
- ^ Banerjee, Ajoy K.; Laya, M. S. (2000). "Reagents for the preparation and cleavage of 1,3-dithiolanes". Russian Chemical Reviews. 69 (11): 947–955. Bibcode:2000RuCRv..69..947B. doi:10.1070/rc2000v069n11abeh000583. S2CID 250918297.