1,2-Dioxin
Appearance
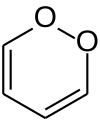
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Dioxine | |||
| Systematic IUPAC name
1,2-Dioxacyclohexa-3,5-diene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H4O2 | |||
| Molar mass | 84.074 g·mol−1 | ||
| Related compounds | |||
Related compounds
|
Dibenzodioxin | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
1,2-Dioxin is a heterocyclic, organic, antiaromatic[1] compound with the chemical formula C4H4O2. It is an isomeric form of 1,4-dioxin (or p-dioxin).
Due to its peroxide-like characteristics, 1,2-dioxin is very unstable and has not been isolated. Calculations suggest that it would isomerize rapidly into but-2-enedial.[2] Even substituted derivatives are very labile, e.g. 1,4-diphenyl-2,3-benzodioxin.[3] Indeed, in 1990, 3,6-bis(p-tolyl)-1,2-dioxin was wrongly accounted as the first stable derivative.[4] It was subsequently shown that the initial compound was not a derivative of 1,2-dioxin, but a thermodynamically more stable dione.[5]
-
The isomers 1,2-dioxin (left) and 1,4-dioxin (right)
-
Structure of the transient 1,4-diphenyl- 2,3-benzodioxin
-
Dioxin (1) and dione form (2)
References
[edit]- ^ Pelloni, Stefano; Faglioni, Francesco; Lazzeretti, Paolo (2013-09-01). "Parity violation energies of C4H4X2 molecules for X = O, S, Se, Te and Po". Molecular Physics. 111 (16–17): 2387–2391. doi:10.1080/00268976.2013.794396. ISSN 0026-8976. S2CID 100861633.
- ^ Matsumoto, M. (2014). "Product Class 1: 1,2-Dioxins and Benzo- and Dibenzo- Fused Derivatives". Science of Synthesis: Houben-Weyl Methods of Molecular Transformations Vol. 16: Six-Membered Hetarenes with Two Identical Heteroatoms. Georg Thieme Verlag. p. 13. ISBN 9783131718815. Retrieved 2020-06-12.
- ^ Smith, Jimmie P.; Schrock, Alan K.; Schuster, Gary B. (1982). "Chemiluminescence of organic peroxides. Thermal generation of an o-xylylene peroxide". Journal of the American Chemical Society. 104 (4): 1041. doi:10.1021/ja00368a021..
- ^ Shine, Henry J.; Zhao, Da Chuan (1990). "Electron transfer to excited doublet states. Photoirradiation of 10-methylphenothiazine cation radical perchlorate in solutions of phenylacetylene and p-tolylacetylene in acetonitrile". The Journal of Organic Chemistry. 55 (13): 4086. doi:10.1021/jo00300a026..
- ^ Block, Eric; Shan, Zhixing; Glass, Richard S.; Fabian, Jürgen (2003). "Revised Structure of a Purported 1,2-Dioxin: A Combined Experimental and Theoretical Study". The Journal of Organic Chemistry. 68 (10): 4108–11. doi:10.1021/jo034305i. PMID 12737603.