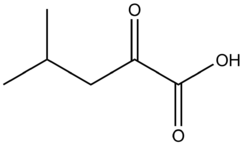
α-Ketoisocaproic acid
| |
| Names | |
|---|---|
| IUPAC name
4-Methyl-2-oxopentanoic acid
| |
| Systematic IUPAC name
4-Methyl-2-oxopentanoic acid[1] | |
| Other names
4-Methyl-2-oxovaleric acid
2-Ketoisocaproic acid 2-Oxo-4-methylpentanoic acid 2-Oxo-4-methylvaleric acid 2-Oxoisocaproic acid 2-Oxoleucine Isobutylglyoxylic acid Ketoleucine α-Ketoisocapronic acid α-Oxoisocaproic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1701823 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.011.304 |
| EC Number |
|
| KEGG | |
| MeSH | Alpha-ketoisocaproic+acid |
PubChem CID
|
|
| UNII | |
| UN number | 3265 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H10O3 | |
| Molar mass | 130.143 g·mol−1 |
| Density | 1.055 g cm−3 (at 20 °C) |
| Melting point | 8 to 10 °C (46 to 50 °F; 281 to 283 K) |
| Boiling point | 85 °C (185 °F; 358 K) at 13 mmHg |
| log P | 0.133 |
| Acidity (pKa) | 2.651 |
| Basicity (pKb) | 11.346 |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
α-Ketoisocaproic acid (α-KIC), also known as 4-methyl-2-oxovaleric acid, and its conjugate base and carboxylate, α-ketoisocaproate, are metabolic intermediates in the metabolic pathway for L-leucine.[2] Leucine is an essential amino acid, and its degradation is critical for many biological duties.[3] α-KIC is produced in one of the first steps of the pathway by branched-chain amino acid aminotransferase by transferring the amine on L-leucine onto alpha ketoglutarate, and replacing that amine with a ketone. The degradation of L-leucine in the muscle to this compound allows for the production of the amino acids alanine and glutamate as well. In the liver, α-KIC can be converted to a vast number of compounds depending on the enzymes and cofactors present, including cholesterol, acetyl-CoA, isovaleryl-CoA, and other biological molecules. Isovaleryl-CoA is the main compound synthesized from ɑ-KIC.[2][4][5] α-KIC is a key metabolite present in the urine of people with Maple syrup urine disease, along with other branched-chain amino acids.[6] Derivatives of α-KIC have been studied in humans for their ability to improve physical performance during anaerobic exercise as a supplemental bridge between short-term and long-term exercise supplements. These studies show that α-KIC does not achieve this goal without other ergogenic supplements present as well.[7] α-KIC has also been observed to reduce skeletal muscle damage after eccentrically biased resistance exercises in people who do not usually perform those exercises.[8]
Biological activity
[edit]Supplements
[edit]α-KIC has been studied as a nutritional supplement to aid in the performance of strenuous physical activity. Studies have shown that taking ɑ-KIC and its derivatives before acute physical activity led to an increase in muscle work by 10%, as well as a decrease in muscle fatigue during the early phase of the physical activity.[7] When taken with other supplements over a two-week period, such as beta-hydroxy beta-methylbutyrate (HMB), participants reported delayed onset of muscle soreness, as well as other positive effects such as increased muscle girth.[8] It is important to note that studies have also suggested that ɑ-KIC taken alone did not have any significant positive impacts on physical performance, so it should be taken in conjunction with other ergogenic substances.[9] ɑ-KIC is not available as a supplement on its own, but its decarboxylated form HMB is available in calcium salt capsules or powder.[2]
Applications
[edit]The biochemical implications of α-KIC are largely connected to other biochemical pathways. Protein Synthesis, skeletal muscle regeneration, and skeletal muscle proteolysis have all been noted to change when ɑ-KIC is taken. There is not much research into the specific mechanisms taking part in these processes, but there is a noticeable correlation between ɑ-KIC ingestion and increased skeletal muscle protein synthesis, regeneration, and proteolysis.[2]
Toxicity
[edit]Multiple studies have demonstrated that there have been no adverse effects on humans nor animals that ingested α-KIC or HMB.[10][11]
In patients with maple syrup urine disease, who are unable to metabolize the branched chain alpha keto acids, α-KIC is believed to be one of the key mediators of neurotoxicity.[12]
Medical use
[edit]Branched-chain alpha-keto acids such as α-KIC are found in high concentrations in the urine of people who suffer from Maple Syrup Urine Disease. This is disease is caused by a partial branched-chain alpha-keto acid dehydrogenase deficiency, which leads to a buildup of branched-chain alpha-keto acids, including α-KIC and HMB.[13] These keto-acids build up in the liver,[2][4][5] and since limited isovaleryl-CoA can be produced, these keto-acids must be excreted in the urine as α-KIC, HMB, and many other similar keto acids. Flare-ups in people who have this condition are caused due to poor diet.[6] Symptoms of Maple Syrup Urine Disease include sweet smelling urine, irritability, lethargy, and in serious cases edema of the brain, apnea, coma, or respiratory failure.[13][6] Treatment includes lowering leucine intake and a specialized diet to make up for the lack of leucine ingestion.[6]
Leucine metabolism
[edit]References
[edit]- ^ CID 70 from PubChem
- ^ a b c d e Wilson, Jacob M.; Fitschen, Peter J.; Campbell, Bill; Wilson, Gabriel J.; Zanchi, Nelo; Taylor, Lem; Wilborn, Colin; Kalman, Douglas S.; Stout, Jeffrey R.; Hoffman, Jay R.; Ziegenfuss, Tim N.; Lopez, Hector L.; Kreider, Richard B.; Smith-Ryan, Abbie E.; Antonio, Jose (2 February 2013). "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". Journal of the International Society of Sports Nutrition. 10 (1): 6. doi:10.1186/1550-2783-10-6. PMC 3568064. PMID 23374455.
- ^ "Leucine".
- ^ a b Zanchi, Nelo Eidy; Gerlinger-Romero, Frederico; Guimarães-Ferreira, Lucas; de Siqueira Filho, Mário Alves; Felitti, Vitor; Lira, Fabio Santos; Seelaender, Marília; Lancha, Antonio Herbert (April 2011). "HMB supplementation: clinical and athletic performance-related effects and mechanisms of action". Amino Acids. 40 (4): 1015–1025. doi:10.1007/s00726-010-0678-0. PMID 20607321. S2CID 11120110.
- ^ a b Kohlmeier, M (May 2015). "Leucine". Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. pp. 385–388. ISBN 978-0-12-387784-0.
- ^ a b c d Strauss, Kevin A; Puffenberger, Erik G; Carson, Vincent J (1993). "Maple Syrup Urine Disease". GeneReviews. University of Washington, Seattle.
- ^ a b Stevens, Bruce R. (2013). "An Overview of Glycine-Arginine-Alpha-Ketoisocaproic Acid (GAKIC) in Sports Nutrition". Nutrition and Enhanced Sports Performance. pp. 433–438. doi:10.1016/B978-0-12-396454-0.00044-8. ISBN 978-0-12-396454-0.
- ^ a b Someren, Ken A. van; Edwards, Adam J.; Howatson, Glyn (1 August 2005). "Supplementation with β-Hydroxy- β-Methylbutyrate (HMB) and α-Ketoisocaproic Acid (KIC) Reduces Signs and Symptoms of Exercise-Induced Muscle Damage in Man". International Journal of Sport Nutrition and Exercise Metabolism. 15 (4): 413–424. doi:10.1123/ijsnem.15.4.413. PMID 16286672.
- ^ Yarrow, Joshua F; Parr, Jeffrey J; White, Lesley J; Borsa, Paul A; Stevens, Bruce R (December 2007). "The effects of short-term alpha-ketoisocaproic acid supplementation on exercise performance: a randomized controlled trial". Journal of the International Society of Sports Nutrition. 4 (1): 2. doi:10.1186/1550-2783-4-2. PMC 2042499. PMID 17908285.
- ^ Nissen, S.; Sharp, R. L.; Panton, L.; Vukovich, M.; Trappe, S.; Fuller, J. C. (1 August 2000). "β-Hydroxy-β-Methylbutyrate (HMB) Supplementation in Humans Is Safe and May Decrease Cardiovascular Risk Factors". The Journal of Nutrition. 130 (8): 1937–1945. doi:10.1093/jn/130.8.1937. PMID 10917905.
- ^ Baxter, J.H.; Carlos, J.L.; Thurmond, J.; Rehani, R.N.; Bultman, J.; Frost, D. (December 2005). "Dietary toxicity of calcium β-hydroxy-β-methyl butyrate (CaHMB)". Food and Chemical Toxicology. 43 (12): 1731–1741. doi:10.1016/j.fct.2005.05.016. PMID 16006030.
- ^ Zinnanti, William J.; Lazovic, Jelena (2012). "Interrupting the mechanisms of brain injury in a model of maple syrup urine disease encephalopathy". Journal of Inherited Metabolic Disease. 35 (1): 71–79. doi:10.1007/s10545-011-9333-5. PMID 21541722. S2CID 1253267.
- ^ a b Strauss, Kevin A.; Wardley, Bridget; Robinson, Donna; Hendrickson, Christine; Rider, Nicholas L.; Puffenberger, Erik G.; Shelmer, Diana; Moser, Ann B.; Morton, D. Holmes (April 2010). "Classical maple syrup urine disease and brain development: Principles of management and formula design". Molecular Genetics and Metabolism. 99 (4): 333–345. doi:10.1016/j.ymgme.2009.12.007. PMC 3671925. PMID 20061171.
- ^ a b Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J (February 2013). "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". Journal of the International Society of Sports Nutrition. 10 (1): 6. doi:10.1186/1550-2783-10-6. PMC 3568064. PMID 23374455.
- ^ a b Kohlmeier M (May 2015). "Leucine". Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. pp. 385–388. ISBN 978-0-12-387784-0. Retrieved 6 June 2016.
Energy fuel: Eventually, most Leu is broken down, providing about 6.0kcal/g. About 60% of ingested Leu is oxidized within a few hours ... Ketogenesis: A significant proportion (40% of an ingested dose) is converted into acetyl-CoA and thereby contributes to the synthesis of ketones, steroids, fatty acids, and other compounds
Figure 8.57: Metabolism of L-leucine