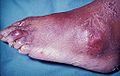
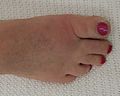
Gout
| Gout | |
|---|---|
| Other names | Arthritis uratica, or Podagra when of the foot |
 | |
| Medical animation of the big toe joint affected by gout | |
| Specialty | Rheumatology |
| Symptoms | Joint pain, swelling, and redness[1] |
| Usual onset | Older males,[1] postmenopausal women[2] |
| Causes | Uric acid[2] |
| Risk factors | Diet high in meat or beer, being overweight, genetics[1][3] |
| Differential diagnosis | Joint infection, rheumatoid arthritis, pseudogout, others[4] |
| Prevention | Weight loss, abstinence from drinking alcohol, allopurinol[5] |
| Treatment | NSAIDs, glucocorticoids, colchicine[2][6] |
| Frequency | 1–2% (developed world)[5] |
Gout (/ɡaʊt/ GOWT[7]) is a form of inflammatory arthritis characterized by recurrent attacks of pain in a red, tender, hot, and swollen joint,[2][8] caused by the deposition of needle-like crystals of uric acid known as monosodium urate crystals.[9] Pain typically comes on rapidly, reaching maximal intensity in less than 12 hours.[5] The joint at the base of the big toe is affected (Podagra) in about half of cases.[10][11] It may also result in tophi, kidney stones, or kidney damage.[1]
Gout is due to persistently elevated levels of uric acid (urate) in the blood (hyperuricemia).[2][5] This occurs from a combination of diet, other health problems, and genetic factors.[1][2] At high levels, uric acid crystallizes and the crystals deposit in joints, tendons, and surrounding tissues, resulting in an attack of gout.[1] Gout occurs more commonly in those who regularly drink beer or sugar-sweetened beverages; eat foods that are high in purines such as liver, shellfish, or anchovies; or are overweight.[1][3] Diagnosis of gout may be confirmed by the presence of crystals in the joint fluid or in a deposit outside the joint.[1] Blood uric acid levels may be normal during an attack.[1]
Treatment with nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, or colchicine improves symptoms.[1][2][12] Once the acute attack subsides, levels of uric acid can be lowered via lifestyle changes and in those with frequent attacks, allopurinol or probenecid provides long-term prevention.[5] Taking vitamin C and having a diet high in low-fat dairy products may be preventive.[13][14]
Gout affects about 1–2% of adults in the developed world at some point in their lives.[5] It has become more common in recent decades.[1] This is believed to be due to increasing risk factors in the population, such as metabolic syndrome, longer life expectancy, and changes in diet.[5] Older males are most commonly affected.[1] Gout was historically known as "the disease of kings" or "rich man's disease".[5][15] It has been recognized at least since the time of the ancient Egyptians.[5]
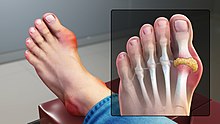
Signs and symptoms
[edit]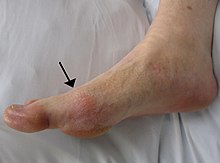
Gout can present in several ways, although the most common is a recurrent attack of acute inflammatory arthritis (a red, tender, hot, swollen joint).[4] The metatarsophalangeal joint at the base of the big toe is affected most often, accounting for half of cases.[10] Other joints, such as the heels, knees, wrists, and fingers, may also be affected.[4] Joint pain usually begins during the night and peaks within 24 hours of onset.[4] This is mainly due to lower body temperature.[1] Other symptoms may rarely occur along with the joint pain, including fatigue and high fever.[10][16]
Long-standing elevated uric acid levels (hyperuricemia) may result in other symptoms, including hard, painless deposits of uric acid crystals called tophi. Extensive tophi may lead to chronic arthritis due to bone erosion.[17] Elevated levels of uric acid may also lead to crystals precipitating in the kidneys, resulting in kidney stone formation and subsequent acute uric acid nephropathy.[18]
Cause
[edit]
The crystallization of uric acid, often related to relatively high levels in the blood, is the underlying cause of gout. This can occur because of diet, genetic predisposition, or underexcretion of urate, the salts of uric acid.[1] Underexcretion of uric acid by the kidney is the primary cause of hyperuricemia in about 90% of cases, while overproduction is the cause in less than 10%.[5] About 10% of people with hyperuricemia develop gout at some point in their lifetimes.[19] The risk, however, varies depending on the degree of hyperuricemia. When levels are between 415 and 530 μmol/L (7 and 8.9 mg/dL), the risk is 0.5% per year, while in those with a level greater than 535 μmol/L (9 mg/dL), the risk is 4.5% per year.[16]
Lifestyle
[edit]Dietary causes account for about 12% of gout,[20] and include a strong association with the consumption of alcohol, sugar-sweetened beverages,[21] meat, and seafood.[4] Among foods richest in purines yielding high amounts of uric acid are dried anchovies, shrimp, organ meat, dried mushrooms, seaweed, and beer yeast.[22] Chicken and potatoes also appear related.[23] Other triggers include physical trauma and surgery.[5]
Studies in the early 2000s found that other dietary factors are not relevant.[24][25] Specifically, a diet with moderate purine-rich vegetables (e.g., beans, peas, lentils, and spinach) is not associated with gout.[26] Neither is total dietary protein.[25][26] Alcohol consumption is strongly associated with increased risk, with wine presenting somewhat less of a risk than beer or spirits.[26][27] Eating skim milk powder enriched with glycomacropeptide (GMP) and G600 milk fat extract may reduce pain but may result in diarrhea and nausea.[28]
Physical fitness, healthy weight, low-fat dairy products, and to a lesser extent, coffee and taking vitamin C, appear to decrease the risk of gout;[29][30][31][32] however, taking vitamin C supplements does not appear to have a significant effect in people who already have established gout.[1] Peanuts, brown bread, and fruit also appear protective.[23] This is believed to be partly due to their effect in reducing insulin resistance.[31]
Other than dietary and lifestyle choices, the recurrence of gout attacks is also linked to the weather. High ambient temperature and low relative humidity may increase the risk of a gout attack.[33]
Genetics
[edit]Gout is partly genetic, contributing to about 60% of variability in uric acid level.[5] The SLC2A9, SLC22A12, and ABCG2 genes have been found to be commonly associated with gout and variations in them can approximately double the risk.[34][35] Loss-of-function mutations in SLC2A9 and SLC22A12 causes low blood uric acid levels by reducing urate absorption and unopposed urate secretion.[35] The rare genetic disorders familial juvenile hyperuricemic nephropathy, medullary cystic kidney disease, phosphoribosylpyrophosphate synthetase superactivity and hypoxanthine-guanine phosphoribosyltransferase deficiency as seen in Lesch–Nyhan syndrome, are complicated by gout.[5]
Medical conditions
[edit]Gout frequently occurs in combination with other medical problems. Metabolic syndrome, a combination of abdominal obesity, hypertension, insulin resistance, and abnormal lipid levels, occurs in nearly 75% of cases.[10] Other conditions commonly complicated by gout include lead poisoning, kidney failure, hemolytic anemia, psoriasis, solid organ transplants, and myeloproliferative disorders such as polycythemia.[5][36] A body mass index greater than or equal to 35 increases male risk of gout threefold.[24] Chronic lead exposure and lead-contaminated alcohol are risk factors for gout due to the harmful effect of lead on kidney function.[37]
Medication
[edit]Diuretics have been associated with attacks of gout, but a low dose of hydrochlorothiazide does not seem to increase risk.[38] Other medications that increase the risk include niacin, aspirin (acetylsalicylic acid), ACE inhibitors, angiotensin receptor blockers, beta blockers, ritonavir, and pyrazinamide.[1][17] The immunosuppressive drugs ciclosporin and tacrolimus are also associated with gout,[5] the former more so when used in combination with hydrochlorothiazide.[39] Hyperuricemia may be induced by excessive use of Vitamin D supplements. Levels of serum uric acid have been positively associated with 25(OH) D. The incidence of hyperuricemia increased 9.4% for every 10 nmol/L increase in 25(OH) D (P < 0.001).[40]
Pathophysiology
[edit]
Gout is a disorder of purine metabolism,[5] and occurs when its final metabolite, uric acid, crystallizes in the form of monosodium urate, precipitating and forming deposits (tophi) in joints, on tendons, and in the surrounding tissues.[17] Microscopic tophi may be walled off by a ring of proteins, which blocks interaction of the crystals with cells and therefore avoids inflammation.[41] Naked crystals may break out of walled-off tophi due to minor physical damage to the joint, medical or surgical stress, or rapid changes in uric acid levels.[41] When they break through the tophi, they trigger a local immune-mediated inflammatory reaction in macrophages, which is initiated by the NLRP3 inflammasome protein complex.[1][17][41] Activation of the NLRP3 inflammasome recruits the enzyme caspase 1, which converts pro-interleukin 1β into active interleukin 1β, one of the key proteins in the inflammatory cascade.[1] An evolutionary loss of urate oxidase (uricase), which breaks down uric acid, in humans and higher primates has made this condition common.[5]
The triggers for precipitation of uric acid are not well understood. While it may crystallize at normal levels, it is more likely to do so as levels increase.[17][42] Other triggers believed to be important in acute episodes of arthritis include cool temperatures, rapid changes in uric acid levels, acidosis, articular hydration and extracellular matrix proteins.[5][43][44] The increased precipitation at low temperatures partly explains why the joints in the feet are most commonly affected.[20] Rapid changes in uric acid may occur due to factors including trauma, surgery, chemotherapy and diuretics.[16] The starting or increasing of urate-lowering medications can lead to an acute attack of gout with febuxostat of a particularly high risk.[45] Calcium channel blockers and losartan are associated with a lower risk of gout compared to other medications for hypertension.[46]
Diagnosis
[edit]| Type | WBC (per mm3) | % neutrophils | Viscosity | Appearance |
|---|---|---|---|---|
| Normal | <200 | 0 | High | Transparent |
| Osteoarthritis | <5000 | <25 | High | Clear yellow |
| Trauma | <10,000 | <50 | Variable | Bloody |
| Inflammatory | 2,000–50,000 | 50–80 | Low | Cloudy yellow |
| Septic arthritis | >50,000 | >75 | Low | Cloudy yellow |
| Gonorrhea | ~10,000 | 60 | Low | Cloudy yellow |
| Tuberculosis | ~20,000 | 70 | Low | Cloudy yellow |
| Inflammatory: Arthritis, gout, rheumatoid arthritis, rheumatic fever | ||||
Gout may be diagnosed and treated without further investigations in someone with hyperuricemia and the classic acute arthritis of the base of the great toe (known as podagra). Synovial fluid analysis should be done if the diagnosis is in doubt.[16][49] Plain X-rays are usually normal and are not useful for confirming a diagnosis of early gout.[5] They may show signs of chronic gout such as bone erosion.[45]
Synovial fluid
[edit]A definitive diagnosis of gout is based upon the identification of monosodium urate crystals in synovial fluid or a tophus.[4] All synovial fluid samples obtained from undiagnosed inflamed joints by arthrocentesis should be examined for these crystals.[5] Under polarized light microscopy, they have a needle-like morphology and strong negative birefringence. This test is difficult to perform and requires a trained observer.[50] The fluid must be examined relatively soon after aspiration, as temperature and pH affect solubility.[5]
Blood tests
[edit]Hyperuricemia is a classic feature of gout, but nearly half of the time gout occurs without hyperuricemia and most people with raised uric acid levels never develop gout.[10][51] Thus, the diagnostic utility of measuring uric acid levels is limited.[10] Hyperuricemia is defined as a plasma urate level greater than 420 μmol/L (7.0 mg/dL) in males and 360 μmol/L (6.0 mg/dL) in females.[52] Other blood tests commonly performed are white blood cell count, electrolytes, kidney function and erythrocyte sedimentation rate (ESR). However, both the white blood cells and ESR may be elevated due to gout in the absence of infection.[53][54] A white blood cell count as high as 40.0×109/l (40,000/mm3) has been documented.[16]
Differential diagnosis
[edit]The most important differential diagnosis in gout is septic arthritis.[5][10] This should be considered in those with signs of infection or those who do not improve with treatment.[10] To help with diagnosis, a synovial fluid Gram stain and culture may be performed.[10] Other conditions that can look similar include CPPD (pseudogout), rheumatoid arthritis, psoriatic arthritis, palindromic rheumatism, and reactive arthritis.[1][10] Gouty tophi, in particular when not located in a joint, can be mistaken for basal cell carcinoma[55] or other neoplasms.[56]
-
Light microscopy of a touch preparation of a gout tophus, showing needle-shaped crystals.
-
Uric acid crystals in polarized light, showing negative birefringence, with yellow color when aligned parallel to the axis of the red compensator, and blue when aligned perpendicularly to it.[57]
-
In contrast, CPPD (pseudogout) displays rhombus-shaped crystals with positive birefringence.
-
Gout on X-rays of a left foot in the metatarsal-phalangeal joint of the big toe. Note also the soft tissue swelling at the lateral border of the foot.
Prevention
[edit]Risk of gout attacks can be lowered by complete abstinence from drinking alcoholic beverages, reducing the intake of fructose (e.g. high fructose corn syrup),[58] sucrose, and purine-rich foods of animal origin, such as organ meats and seafood.[3] Eating dairy products, vitamin C-rich foods, coffee, and cherries may help prevent gout attacks, as does losing weight.[3][59] Gout may be secondary to sleep apnea via the release of purines from oxygen-starved cells. Treatment of apnea can lessen the occurrence of attacks.[60]
Medications
[edit]As of 2020, allopurinol is generally the recommended preventative treatment if medications are used.[61][62] A number of other medications may occasionally be considered to prevent further episodes of gout, including probenecid, febuxostat, benzbromarone, and colchicine.[12][63][64] Long term medications are not recommended until a person has had two attacks of gout,[20] unless destructive joint changes, tophi, or urate nephropathy exist.[18] It is not until this point that medications are cost-effective.[20] They are not usually started until one to two weeks after an acute flare has resolved, due to theoretical concerns of worsening the attack.[20] They are often used in combination with either an NSAID or colchicine for the first three to six months.[5][12]
While it has been recommended that urate-lowering measures should be increased until serum uric acid levels are below 300–360 μmol/L (5.0–6.0 mg/dL),[61][65] there is little evidence to support this practice over simply putting people on a standard dose of allopurinol.[66] If these medications are in chronic use at the time of an attack, it is recommended that they be continued.[10] Levels that cannot be brought below 6.0 mg/dL while attacks continue indicates refractory gout.[67]
While historically it is not recommended to start allopurinol during an acute attack of gout, this practice appears acceptable.[68] Allopurinol blocks uric acid production, and is the most commonly used agent.[20] Long term therapy is safe and well-tolerated and can be used in people with renal impairment or urate stones, although hypersensitivity occurs in a small number of individuals.[20] The HLA-B*58:01 allele of the human leukocyte antigen B (HLA-B) is strongly associated with severe cutaneous adverse reactions during treatment with allopurinol and is most common among Asian subpopulations, notably those of Korean, Han-Chinese, or Thai descent.[69]
Febuxostat is only recommended in those who cannot tolerate allopurinol.[70] There are concerns about more deaths with febuxostat compared to allopurinol.[71] Febuxostat may also increase the rate of gout flares during early treatment.[72] However, there is tentative evidence that febuxostat may bring down urate levels more than allopurinol.[73]
Probenecid appears to be less effective than allopurinol and is a second line agent.[20][63] Probenecid may be used if undersecretion of uric acid is present (24-hour urine uric acid less than 800 mg).[74] It is, however, not recommended if a person has a history of kidney stones.[74] Probenecid can be used in a combined therapy with allopurinol is more effective than allopurinol monotherapy.[75][76][77]
Pegloticase is an option for the 3% of people who are intolerant to other medications.[78] It is a third line agent.[63] Pegloticase is given as an intravenous infusion every two weeks,[78] and reduces uric acid levels.[79] Pegloticase is useful decreasing tophi but has a high rate of side effects and many people develop resistance to it.[63] Using lesinurad 400 mg plus febuxostat is more beneficial for tophi resolution than lesinural 200 mL with febuxostat, with similar side effects. Lesinural plus allopurinol is not effective for tophi resolution.[80] Potential side effects include kidney stones, anemia and joint pain.[81] In 2016, it was withdrawn from the European market.[82][83]
Lesinurad reduces blood uric acid levels by preventing uric acid absorption in the kidneys.[84] It was approved in the United States for use together with allopurinol, among those who were unable to reach their uric acid level targets.[85] Side effects include kidney problems and kidney stones.[84][86]
Treatment
[edit]The initial aim of treatment is to settle the symptoms of an acute attack.[87] Repeated attacks can be prevented by medications that reduce serum uric acid levels.[87] Tentative evidence supports the application of ice for 20 to 30 minutes several times a day to decrease pain.[88] Options for acute treatment include nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and glucocorticoids.[20] While glucocorticoids and NSAIDs work equally well, glucocorticoids may be safer.[89] Options for prevention include allopurinol, febuxostat, and probenecid. Lowering uric acid levels can cure the disease.[5] Treatment of associated health problems is also important.[5] Lifestyle interventions have been poorly studied.[88] It is unclear whether dietary supplements have an effect in people with gout.[90]
NSAIDs
[edit]NSAIDs are the usual first-line treatment for gout. No specific agent is significantly more or less effective than any other.[20] Improvement may be seen within four hours and treatment is recommended for one to two weeks.[5][20] They are not recommended for those with certain other health problems, such as gastrointestinal bleeding, kidney failure, or heart failure.[91] While indometacin has historically been the most commonly used NSAID, an alternative, such as ibuprofen, may be preferred due to its better side effect profile in the absence of superior effectiveness.[38] For those at risk of gastric side effects from NSAIDs, an additional proton pump inhibitor may be given.[92] There is some evidence that COX-2 inhibitors may work as well as nonselective NSAIDs for acute gout attack with fewer side effects.[93][94][95][96]
Colchicine
[edit]Colchicine is an alternative for those unable to tolerate NSAIDs.[20] At high doses, side effects (primarily gastrointestinal upset) limit its usage.[97] At lower doses, which are still effective, it is well tolerated.[38][98][95][96] Colchicine may interact with other commonly prescribed drugs, such as atorvastatin and erythromycin, among others.[97]
Glucocorticoids
[edit]Glucocorticoids have been found to be as effective as NSAIDs[94][99] and may be used if contraindications exist for NSAIDs.[20][100] They also lead to improvement when injected into the joint.[20] A joint infection must be excluded, however, as glucocorticoids worsen this condition.[20] There were no short-term adverse effects reported.[101]
Others
[edit]Interleukin-1 inhibitors, such as canakinumab, showed moderate effectiveness for pain relief and reduction of joint swelling, but have increased risk of adverse events, such as back pain, headache, and increased blood pressure.[102] They, however, may work less well than usual doses of NSAIDS.[102] The high cost of this class of drugs may also discourage their use for treating gout.[102]
Prognosis
[edit]Without treatment, an acute attack of gout usually resolves in five to seven days; however, 60% of people have a second attack within one year.[16] Those with gout are at increased risk of hypertension, diabetes mellitus, metabolic syndrome, and kidney and cardiovascular disease and thus are at increased risk of death.[5][103] It is unclear whether medications that lower urate affect cardiovascular disease risks.[104] This may be partly due to its association with insulin resistance and obesity, but some of the increased risk appears to be independent.[103]
Without treatment, episodes of acute gout may develop into chronic gout with destruction of joint surfaces, joint deformity, and painless tophi.[5] These tophi occur in 30% of those who are untreated for five years, often in the helix of the ear, over the olecranon processes, or on the Achilles tendons.[5] With aggressive treatment, they may dissolve. Kidney stones also frequently complicate gout, affecting between 10 and 40% of people, and occur due to low urine pH promoting the precipitation of uric acid.[5] Other forms of chronic kidney dysfunction may occur.[5]
-
Gouty tophi presenting as nodules on the finger and helix of the ear
-
Tophus of the knee
-
Tophii on the toe and ankle
-
Gout complicated by ruptured tophi, the exudate of which tested positive for uric acid crystals
-
Gout in the big toe of left foot, compared to the healthy right foot
-
Gout in the joint of the big toe
-
Gross pathology of a large tophus
Epidemiology
[edit]Gout affects around 1–2% of people in the Western world at some point in their lifetimes and is becoming more common.[5][20] Some 5.8 million people were affected in 2013.[105] Rates of gout approximately doubled between 1990 and 2010.[17] This rise is believed to be due to increasing life expectancy, changes in diet and an increase in diseases associated with gout, such as metabolic syndrome and high blood pressure.[24] Factors that influence rates of gout include age, race, and the season of the year. In men over 30 and women over 50, rates are 2%.[91]
In the United States, gout is twice as likely in males of African descent than those of European descent.[106] Rates are high among Polynesians, but the disease is rare in aboriginal Australians, despite a higher mean uric acid serum concentration in the latter group.[107] It has become common in China, Polynesia, and urban Sub-Saharan Africa.[5] Some studies found that attacks of gout occur more frequently in the spring. This has been attributed to seasonal changes in diet, alcohol consumption, physical activity, and temperature.[108]
Taiwan, Hong Kong and Singapore have releatively higher prevalence of gout. A study based on the National Health Insurance Research Database (NHIRD) estimated that 4.92% of Taiwanese residents have gout in 2004. A survey hold by the Hong Kong government found that 5.1% of Hong Kong resident between 45–59 years and 6.1% of those older than 60 years have gout. A study hold in Singapore found that 2,117 in 52,322 people between 45–74 years have gout, roughly equals to 4.1%.[109]
History
[edit]
The English term "gout" first occurs in the work of Randolphus of Bocking, around 1200 AD.[111] It derives from the Latin word gutta, meaning "a drop" (of liquid).[110] According to the Oxford English Dictionary, this originates from humorism and "the notion of the 'dropping' of a morbid material from the blood in and around the joints".[112]
Gout has been known since antiquity. Historically, wits have referred to it as "the king of diseases and the disease of kings"[5][113] or as "rich man's disease".[15] The Ebers papyrus and the Edwin Smith papyrus, (c. 1550 BC) each mention arthritis of the first metacarpophalangeal joint as a distinct type of arthritis. These ancient manuscripts cite (now missing) Egyptian texts about gout that are claimed to have been written 1,000 years earlier and ascribed to Imhotep.[114] Greek physician Hippocrates around 400 BC commented on it in his Aphorisms, noting its absence in eunuchs and premenopausal women.[110][115] Aulus Cornelius Celsus (30 AD) described the linkage with alcohol, later onset in women and associated kidney problems:
Again thick urine, the sediment from which is white, indicates that pain and disease are to be apprehended in the region of joints or viscera... Joint troubles in the hands and feet are very frequent and persistent, such as occur in cases of podagra and cheiragra. These seldom attack eunuchs or boys before coition with a woman, or women except those in whom the menses have become suppressed... some have obtained lifelong security by refraining from wine, mead and venery.[116]
Benjamin Welles, an English physician, authored the first medical book on gout, A Treatise of the Gout, or Joint Evil, in 1669.[117] In 1683, Thomas Sydenham, an English physician, described its occurrence in the early hours of the morning and its predilection for older males:
Gouty patients are, generally, either old men or men who have so worn themselves out in youth as to have brought on a premature old age—of such dissolute habits none being more common than the premature and excessive indulgence in venery and the like exhausting passions. The victim goes to bed and sleeps in good health. About two o'clock in the morning he is awakened by a severe pain in the great toe; more rarely in the heel, ankle, or instep. The pain is like that of a dislocation and yet parts feel as if cold water were poured over them. Then follows chills and shivers and a little fever... The night is passed in torture, sleeplessness, turning the part affected and perpetual change of posture; the tossing about of body being as incessant as the pain of the tortured joint and being worse as the fit comes on.[118]
In the 18th century, Thomas Marryat distinguished different manifestations of gout:
The Gout is a chronical disease most commonly affecting the feet. If it attacks the knees, it is called Gonagra; if the hands, Chiragra; if the elbow, Onagra; if the shoulder, Omagra; if the back or loins, Lumbago.[119]
Dutch scientist Antonie van Leeuwenhoek first described the microscopic appearance of urate crystals in 1679.[110] In 1848, English physician Alfred Baring Garrod identified excess uric acid in the blood as the cause of gout.[120]
Other animals
[edit]Gout is rare in most other animals due to their ability to produce uricase, which breaks down uric acid.[121] Humans and other great apes do not have this ability; thus, gout is common.[16][121] Other animals with uricase include fish, amphibians and most non-primate mammals.[122] The Tyrannosaurus rex specimen known as "Sue" is believed to have had gout.[123]
Research
[edit]A number of new medications are under study for treating gout, including anakinra, canakinumab, and rilonacept.[124] Canakinumab may result in better outcomes than a low dose of a glucocorticoid, but costs five thousand times more.[125] A recombinant uricase enzyme (rasburicase) is available but its use is limited, as it triggers an immune response. Less antigenic versions are in development.[16]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m n o p q r s Dalbeth N, Merriman TR, Stamp LK (April 2016). "Gout". Lancet (Review). 388 (10055): 2039–2052. doi:10.1016/S0140-6736(16)00346-9. PMID 27112094. S2CID 208790780.
- ^ a b c d e f g Hui M, Carr A, Cameron S, et al. (26 May 2017). "The British Society for Rheumatology Guideline for the Management of Gout". Rheumatology. 56 (7): e1–e20. doi:10.1093/rheumatology/kex156. PMID 28549177.
- ^ a b c d Beyl RN Jr, Hughes L, Morgan S (2016). "Update on Importance of Diet in Gout". The American Journal of Medicine. 129 (11): 1153–1158. doi:10.1016/j.amjmed.2016.06.040. PMID 27452679.
- ^ a b c d e f Neogi T (July 2016). "Gout". Annals of Internal Medicine (Review). 165 (1): ITC1-16. doi:10.7326/AITC201607050. PMID 27380294.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah Richette P, Bardin T (January 2010). "Gout". Lancet. 375 (9711): 318–328. doi:10.1016/S0140-6736(09)60883-7. PMID 19692116. S2CID 208793280.
- ^ Qaseem A, Harris RP, Forciea MA, et al. (3 January 2017). "Management of Acute and Recurrent Gout: A Clinical Practice Guideline From the American College of Physicians". Annals of Internal Medicine. 166 (1): 58–68. doi:10.7326/m16-0570. PMID 27802508.
- ^ "Gout | Definition of Gout by Lexico". Lexico Dictionaries | English. Archived from the original on 19 October 2019. Retrieved 20 October 2019.
- ^ Dalbeth N, Merriman TR, Stamp LK (April 2016). "Gout". Lancet (Review). 388 (10055): 2039–2052. doi:10.1016/S0140-6736(16)00346-9. PMID 27112094. S2CID 208790780.
- ^ Abhishek A, Roddy E, Doherty M (February 2017). "Gout - a guide for the general and acute physicians". Clinical Medicine. 17 (1): 54–59. doi:10.7861/clinmedicine.17-1-54. PMC 6297580. PMID 28148582.
- ^ a b c d e f g h i j k Schlesinger N (March 2010). "Diagnosing and treating gout: a review to aid primary care physicians". Postgrad Med. 122 (2): 157–161. doi:10.3810/pgm.2010.03.2133. PMID 20203467. S2CID 35321485.
- ^ "Definition of Podagra". www.merriam-webster.com. Archived from the original on 19 January 2023. Retrieved 19 January 2023.
- ^ a b c Shekelle PG, Newberry SJ, Fitzgerald JD, et al. (2017). "Management of Gout: A Systematic Review in Support of an American College of Physicians Clinical Practice Guideline". Annals of Internal Medicine. 166 (1): 37–51. doi:10.7326/M16-0461. PMID 27802478.
- ^ "Questions and Answers about Gout". National Institute of Arthritis and Musculoskeletal and Skin Diseases. June 2015. Archived from the original on 15 January 2016. Retrieved 2 February 2016.
- ^ Roddy E, Choi HK (May 2014). "Epidemiology of gout". Rheumatic Disease Clinics of North America. 40 (2): 155–175. doi:10.1016/j.rdc.2014.01.001. PMC 4119792. PMID 24703341.
- ^ a b "Rich Man's Disease – definition of Rich Man's Disease in the Medical dictionary". Free Online Medical Dictionary, Thesaurus and Encyclopedia. Archived from the original on 14 November 2013. Retrieved 1 May 2009.
- ^ a b c d e f g h Eggebeen AT (2007). "Gout: an update". Am Fam Physician. 76 (6): 801–808. PMID 17910294.
- ^ a b c d e f Terkeltaub R (January 2010). "Update on gout: new therapeutic strategies and options". Nature Reviews Rheumatology. 6 (1): 30–38. doi:10.1038/nrrheum.2009.236. PMID 20046204. S2CID 19235998.
- ^ a b Tausche AK, Jansen TL, Schröder HE, et al. (August 2009). "Gout – current diagnosis and treatment". Dtsch Ärztebl Int. 106 (34–35): 549–555. doi:10.3238/arztebl.2009.0549. PMC 2754667. PMID 19795010.
- ^ Vitart V, Rudan I, Hayward C, et al. (April 2008). "SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout". Nat. Genet. 40 (4): 437–442. doi:10.1038/ng.106. PMID 18327257. S2CID 6720464. Archived from the original on 27 July 2022. Retrieved 27 July 2022.
- ^ a b c d e f g h i j k l m n o p Chen LX, Schumacher HR (October 2008). "Gout: an evidence-based review". J Clin Rheumatol. 14 (5 Suppl): S55–S62. doi:10.1097/RHU.0b013e3181896921. PMID 18830092. S2CID 6644013.
- ^ Ebrahimpour-Koujan S, Saneei P, Larijani B, et al. (2020). "Consumption of sugar sweetened beverages and dietary fructose in relation to risk of gout and hyperuricemia: a systematic review and meta-analysis". Crit Rev Food Sci Nutr. 60 (1): 1–10. doi:10.1080/10408398.2018.1503155. PMID 30277800. S2CID 52909165.
- ^ Kaneko K, Aoyagi Y, Fukuuchi T, et al. (2014). "Total Purine and Purine Base Content of Common Foodstuffs for Facilitating Nutritional Therapy for Gout and Hyperuricemia". Biological and Pharmaceutical Bulletin. 37 (5): 709–721. doi:10.1248/bpb.b13-00967. PMID 24553148.
- ^ a b Major TJ, Topless RK, Dalbeth N, et al. (10 October 2018). "Evaluation of the diet wide contribution to serum urate levels: meta-analysis of population based cohorts". BMJ. 363: k3951. doi:10.1136/bmj.k3951. PMC 6174725. PMID 30305269.
- ^ a b c Weaver AL (July 2008). "Epidemiology of gout". Cleveland Clinic Journal of Medicine. 75 (Suppl 5): S9–S12. doi:10.3949/ccjm.75.Suppl_5.S9. PMID 18819329. S2CID 40262260.
- ^ a b Choi HK, Atkinson K, Karlson EW, et al. (March 2004). "Purine-rich foods, dairy and protein intake, and the risk of gout in men". N. Engl. J. Med. 350 (11): 1093–1103. doi:10.1056/NEJMoa035700. PMID 15014182.
- ^ a b c Singh JA, Reddy SG, Kundukulam J (March 2011). "Risk factors for gout and prevention: a systematic review of the literature". Current Opinion in Rheumatology. 23 (2): 192–202. doi:10.1097/BOR.0b013e3283438e13. PMC 4104583. PMID 21285714.
- ^ Roddy E, Mallen CD, Doherty M (1 October 2013). "Gout". BMJ. 347 (oct01 3): f5648. doi:10.1136/bmj.f5648. PMID 24473446. S2CID 220212466.
- ^ Moi JH, Sriranganathan MK, Edwards CJ, et al. (31 May 2013). "Lifestyle interventions for chronic gout". Cochrane Database of Systematic Reviews. 2013 (5): CD010039. doi:10.1002/14651858.cd010039.pub2. ISSN 1465-1858. PMC 6759140. PMID 23728699.
- ^ Hak AE, Choi HK (March 2008). "Lifestyle and gout". Curr Opin Rheumatol. 20 (2): 179–186. doi:10.1097/BOR.0b013e3282f524a2. PMID 18349748. S2CID 205485689.
- ^ Williams PT (May 2008). "Effects of diet, physical activity and performance, and body weight on incident gout in ostensibly healthy, vigorously active men". Am. J. Clin. Nutr. 87 (5): 1480–1487. doi:10.1093/ajcn/87.5.1480. PMC 4090353. PMID 18469274.
- ^ a b Choi HK (March 2010). "A prescription for lifestyle change in patients with hyperuricemia and gout". Curr Opin Rheumatol. 22 (2): 165–172. doi:10.1097/BOR.0b013e328335ef38. PMID 20035225. S2CID 19146212.
- ^ Park KY, Kim HJ, Ahn HS, et al. (April 2016). "Effects of coffee consumption on serum uric acid: systematic review and meta-analysis". Seminars in Arthritis and Rheumatism. 45 (5): 580–586. doi:10.1016/j.semarthrit.2016.01.003. PMID 26905267.
- ^ Neogi T, Chen C, Niu J, et al. (15 August 2014). "Relation of Temperature and Humidity to the Risk of Recurrent Gout Attacks". American Journal of Epidemiology. 180 (4): 372–377. doi:10.1093/aje/kwu147. ISSN 0002-9262. PMC 4184385. PMID 24993733.
- ^ Merriman TR, Dalbeth, N (2011). "The genetic basis of hyperuricaemia and gout". Joint Bone Spine. 78 (1): 35–40. doi:10.1016/j.jbspin.2010.02.027. PMID 20472486.
- ^ a b Reginato AM, Mount DB, Yang I, et al. (2012). "The genetics of hyperuricaemia and gout". Nature Reviews Rheumatology. 8 (10): 610–621. doi:10.1038/nrrheum.2012.144. PMC 3645862. PMID 22945592.
- ^ Stamp L, Searle M, O'Donnell J, et al. (2005). "Gout in solid organ transplantation: a challenging clinical problem". Drugs. 65 (18): 2593–2611. doi:10.2165/00003495-200565180-00004. PMID 16392875. S2CID 46979126.
- ^ Loghman-Adham M (September 1997). "Renal effects of environmental and occupational lead exposure". Environ. Health Perspect. 105 (9): 928–938. doi:10.2307/3433873. JSTOR 3433873. PMC 1470371. PMID 9300927.
- ^ a b c Laubscher T, Dumont Z, Regier L, et al. (December 2009). "Taking the stress out of managing gout". Can Fam Physician. 55 (12): 1209–1212. PMC 2793228. PMID 20008601.
- ^ Firestein GS, Budd RC, Harris ED, McInnes IB, Ruddy S, Sergent JS, eds. (2008). "Chapter 87: Gout and Hyperuricemia". Kelley's Textbook of Rheumatology (8th ed.). Elsevier. ISBN 978-1-4160-4842-8.
- ^ Chen Y (2020). "Association between serum vitamin D and uric acid in the eastern Chinese population: a population-based cross-sectional study". BMC Endocr Disord. 20 (79): 79. doi:10.1186/s12902-020-00560-1. PMC 7268462. PMID 32493273. Archived from the original on 10 July 2024. Retrieved 21 June 2021.
- ^ a b c Liu-Bryan R, Terkeltaub R (2006). "Evil humors take their Toll as innate immunity makes gouty joints TREM-ble". Arthritis & Rheumatism. 54 (2): 383–386. doi:10.1002/art.21634. PMID 16447213.
- ^ Virsaladze DK, Tetradze LO, Dzhavashvili LV, et al. (2007). "[Levels of uric acid in serum in patients with metabolic syndrome]" [Levels of uric acid in serum in patients with metabolic syndrome]. Georgian Med News (in Russian) (146): 35–37. PMID 17595458.
- ^ Moyer RA, John DS (2003). "Acute gout precipitated by total parenteral nutrition". The Journal of Rheumatology. 30 (4): 849–850. PMID 12672211.
- ^ Halabe A, Sperling O (1994). "Uric acid nephrolithiasis". Mineral and Electrolyte Metabolism. 20 (6): 424–431. PMID 7783706.
- ^ a b "Gout". NICE. Archived from the original on 28 October 2016. Retrieved 22 August 2019.
- ^ Choi HK, Soriano LC, Zhang Y, et al. (2012). "Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study". BMJ. 344: d8190. doi:10.1136/bmj.d8190. PMC 3257215. PMID 22240117.
- ^ Flynn JA, Choi MJ, Wooster DL (2013). Oxford American Handbook of Clinical Medicine. US: OUP. p. 400. ISBN 978-0-19-991494-4.
- ^ Seidman AJ, Limaiem F (2019). "Synovial Fluid Analysis". StatPearls. StatPearls Publishing. PMID 30725799. Retrieved 19 December 2019.
- ^ Qaseem A, McLean RM, Starkey M, et al. (3 January 2017). "Diagnosis of Acute Gout: A Clinical Practice Guideline From the American College of Physicians". Annals of Internal Medicine. 166 (1): 52–57. doi:10.7326/m16-0569. PMID 27802479.
- ^ Schlesinger N (2007). "Diagnosis of gout". Minerva Med. 98 (6): 759–767. PMID 18299687.
- ^ Sturrock R (2000). "Gout. Easy to misdiagnose". BMJ. 320 (7228): 132–133. doi:10.1136/bmj.320.7228.132. PMC 1128728. PMID 10634714.
- ^ Sachs L, Batra KL, Zimmermann B (2009). "Medical implications of hyperuricemia". Med Health R I. 92 (11): 353–355. PMID 19999892.
- ^ "Gout: Differential Diagnoses & Workup – eMedicine Rheumatology". Medscape. 17 January 2019. Archived from the original on 25 July 2010.
- ^ "Gout and Pseudogout: Differential Diagnoses & Workup – eMedicine Emergency Medicine". Medscape. 17 January 2019. Archived from the original on 11 March 2010.
- ^ Jordan DR, Belliveau MJ, Brownstein S, et al. (2008). "Medial canthal tophus". Ophthal Plast Reconstr Surg. 24 (5): 403–404. doi:10.1097/IOP.0b013e3181837a31. PMID 18806664.
- ^ Sano K, Kohakura Y, Kimura K, et al. (March 2009). "Atypical Triggering at the Wrist due to Intratendinous Infiltration of Tophaceous Gout". Hand (N Y). 4 (1): 78–80. doi:10.1007/s11552-008-9120-4. PMC 2654956. PMID 18780009.
- ^ Rothschild BM. "Gout and Pseudogout Workup". Medscape. Archived from the original on 8 October 2020. Retrieved 23 September 2020. Updated: Jun 30, 2020
- ^ Jamnik J, Rehman S, Blanco Mejia S, et al. (October 2016). "Fructose intake and risk of gout and hyperuricemia: a systematic review and meta-analysis of prospective cohort studies". BMJ Open. 6 (10): e013191. doi:10.1136/bmjopen-2016-013191. PMC 5073537. PMID 27697882.
- ^ Bitik B, Öztürk MA (June 2014). "An old disease with new insights: Update on diagnosis and treatment of gout". European Journal of Rheumatology. 1 (2): 72–77. doi:10.5152/eurjrheumatol.2014.021. PMC 5042282. PMID 27708879.
- ^ Abrams B (2009). "Sleep Apnea as a Cause of Gout Flares". The Medscape Journal of Medicine. 11 (1): 3. PMC 2654686. PMID 19295924.
- ^ a b FitzGerald JD, Dalbeth N, Mikuls T, et al. (11 May 2020). "2020 American College of Rheumatology Guideline for the Management of Gout". Arthritis & Rheumatology. 72 (6): 879–895. doi:10.1002/art.41247. hdl:2027.42/155484. PMID 32390306.
- ^ Dakkak M, Lanney H (August 2021). "Management of Gout: Update from the American College of Rheumatology". Am Fam Physician. 104 (2): 209–210. PMID 34383428.
- ^ a b c d Dalbeth N, Merriman TR, Stamp LK (22 October 2016). "Gout". Lancet. 388 (10055): 2039–2052. doi:10.1016/s0140-6736(16)00346-9. PMID 27112094. S2CID 208790780.
- ^ Kydd AS, Seth R, Buchbinder R, et al. (14 November 2014). "Uricosuric medications for chronic gout". Cochrane Database of Systematic Reviews (11): CD010457. doi:10.1002/14651858.cd010457.pub2. ISSN 1465-1858. PMC 11262558. PMID 25392987.
- ^ Ruoff G, Edwards NL (September 2016). "Overview of Serum Uric Acid Treatment Targets in Gout: Why Less Than 6 mg/dL?". Postgraduate Medicine. 128 (7): 706–715. doi:10.1080/00325481.2016.1221732. PMID 27558643.
- ^ Qaseem A, Harris RP, Forciea MA (1 November 2016). "Management of Acute and Recurrent Gout: A Clinical Practice Guideline From the American College of Physicians". Annals of Internal Medicine. 166 (1): 58–68. doi:10.7326/M16-0570. PMID 27802508. S2CID 207538623. Archived from the original on 21 May 2022. Retrieved 16 October 2021.
- ^ Ali S, Lally, EV (November 2009). "Treatment failure gout". Medicine and Health, Rhode Island. 92 (11): 369–371. PMID 19999896.
- ^ Robinson PC, Stamp LK (May 2016). "The management of gout: Much has changed". Australian Family Physician. 45 (5): 299–302. PMID 27166465.
- ^ Dean L, Kane M (2012), Pratt VM, Scott SA, Pirmohamed M, Esquivel B (eds.), "Allopurinol Therapy and HLA-B*58:01 Genotype", Medical Genetics Summaries, Bethesda (MD): National Center for Biotechnology Information (US), PMID 28520356, archived from the original on 15 June 2022, retrieved 29 November 2022
- ^ "Febuxostat for the management of hyperuricaemia in people with gout Guidance and guidelines". www.nice.org.uk. 17 December 2008. Archived from the original on 28 March 2017. Retrieved 28 March 2017.
- ^ "Drug Safety and Availability – FDA adds Boxed Warning for increased risk of death with gout medicine Uloric (febuxostat)". FDA. 21 February 2019. Archived from the original on 23 April 2019. Retrieved 26 February 2019.
- ^ Tayar JH, Lopez-Olivo MA, Suarez-Almazor ME (14 November 2012). "Febuxostat for treating chronic gout". Cochrane Database of Systematic Reviews. 11 (11): CD008653. doi:10.1002/14651858.cd008653.pub2. ISSN 1465-1858. PMC 4058893. PMID 23152264.
- ^ Seth R, Kydd AS, Buchbinder R, et al. (14 October 2014). "Allopurinol for chronic gout". Cochrane Database of Systematic Reviews. 2014 (10): CD006077. doi:10.1002/14651858.cd006077.pub3. ISSN 1465-1858. PMC 8915170. PMID 25314636.
- ^ a b Agabegi ED, Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. p. 251. ISBN 978-0-7817-7153-5. Archived from the original on 10 January 2023. Retrieved 10 January 2016.
- ^ "Archived copy". Archived from the original on 10 July 2024. Retrieved 7 July 2024.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Stocker SL, Williams KM, McLachlan AJ, et al. (2008). "Pharmacokinetic and Pharmacodynamic Interaction between Allopurinol and Probenecid??in Healthy Subjects". Clinical Pharmacokinetics. 47 (2): 111–118. doi:10.2165/00003088-200847020-00004. PMID 18193917. Archived from the original on 7 June 2018. Retrieved 7 July 2024.
- ^ Seth R, Kydd AS, Buchbinder R, et al. (2014). "Allopurinol for chronic gout". The Cochrane Database of Systematic Reviews (10): CD006077. doi:10.1002/14651858.CD006077.pub3. PMC 8915170. PMID 25314636.
- ^ a b "FDA approves new drug for gout". FDA. 14 September 2010. Archived from the original on 17 September 2010.
- ^ Sundy JS, Baraf HS, Yood RA, et al. (17 August 2011). "Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials". JAMA: The Journal of the American Medical Association. 306 (7): 711–720. doi:10.1001/jama.2011.1169. hdl:10342/7960. PMID 21846852.
- ^ Sriranganathan MK, Vinik O, Pardo Pardo J, et al. (11 August 2021). "Interventions for tophi in gout". The Cochrane Database of Systematic Reviews. 2021 (8): CD010069. doi:10.1002/14651858.CD010069.pub3. PMC 8406833. PMID 34379791.
- ^ Anderson A, Singh JA (17 March 2010). "Pegloticase for chronic gout". Cochrane Database of Systematic Reviews. 2010 (3): CD008335. doi:10.1002/14651858.cd008335.pub2. ISSN 1465-1858. PMC 6599816. PMID 20238366.
- ^ "Krystexxa". www.ema.europa.eu. Archived from the original on 28 March 2017. Retrieved 28 March 2017.
- ^ "Pegloticase: withdrawal of its EU marketing authorisation is welcome". Prescrire International. 26 (180): 71. March 2017.
- ^ a b "Zurampic". Drugs.com. 1 January 2018. Archived from the original on 15 October 2018. Retrieved 14 October 2018.
- ^ "Drug Trial Snapshot: Zurampic". US Food and Drug Administration. 22 December 2015. Archived from the original on 8 February 2019. Retrieved 14 October 2018.
- ^ "Zurampic" (PDF). European Medicines Agency. 18 February 2016. Archived (PDF) from the original on 28 August 2021. Retrieved 14 October 2018.
- ^ a b Zhang W, Doherty M, Bardin T, et al. (October 2006). "EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT)". Ann. Rheum. Dis. 65 (10): 1312–1324. doi:10.1136/ard.2006.055269. PMC 1798308. PMID 16707532.
- ^ a b Moi JH, Sriranganathan MK, Edwards CJ, et al. (4 November 2013). "Lifestyle interventions for acute gout". The Cochrane Database of Systematic Reviews. 11 (11): CD010519. doi:10.1002/14651858.CD010519.pub2. PMC 9942538. PMID 24186771.
- ^ Billy CA, Lim RT, Ruospo M, et al. (1 August 2017). "Corticosteroid or Nonsteroidal Antiinflammatory Drugs for the Treatment of Acute Gout: A Systematic Review of Randomized Controlled Trials" (PDF). The Journal of Rheumatology. 45 (1): 128–136. doi:10.3899/jrheum.170137. PMID 28765243. S2CID 8306526. Archived (PDF) from the original on 7 August 2020. Retrieved 7 June 2020.
- ^ Andrés M, Sivera F, Buchbinder R, et al. (12 November 2021). "Dietary supplements for chronic gout". The Cochrane Database of Systematic Reviews. 11 (11): CD010156. doi:10.1002/14651858.CD010156.pub3. ISSN 1469-493X. PMC 8589461. PMID 34767649.
- ^ a b Winzenberg T, Buchbinder R (2009). "Cochrane Musculoskeletal Group review: acute gout. Steroids or NSAIDs? Let this overview from the Cochrane Group help you decide what's best for your patient". J Fam Pract. 58 (7): E1–E4. PMID 19607767.
- ^ Cronstein BN, Terkeltaub R (2006). "The inflammatory process of gout and its treatment". Arthritis Research & Therapy. 8 (Suppl 1): S3. doi:10.1186/ar1908. PMC 3226108. PMID 16820042.
- ^ van Durme CM, Wechalekar MD, Landewé RB (9 June 2015). "Nonsteroidal anti-inflammatory drugs for treatment of acute gout". JAMA. 313 (22): 2276–2277. doi:10.1001/jama.2015.1881. PMID 26057289.
- ^ a b van Durme CM, Wechalekar MD, Landewé RB, et al. (9 December 2021). "Non-steroidal anti-inflammatory drugs for acute gout". The Cochrane Database of Systematic Reviews. 2021 (12): CD010120. doi:10.1002/14651858.CD010120.pub3. ISSN 1469-493X. PMC 8656463. PMID 34882311.
- ^ a b Roddy E, Bajpai R, Forrester H, et al. (1 December 2023). "Safety of colchicine and NSAID prophylaxis when initiating urate-lowering therapy for gout: propensity score-matched cohort studies in the UK Clinical Practice Research Datalink". Annals of the Rheumatic Diseases. 82 (12): 1618–1625. doi:10.1136/ard-2023-224154. ISSN 0003-4967. PMC 10646835. PMID 37788904. Archived from the original on 16 February 2024. Retrieved 16 February 2024.
- ^ a b "How common are side-effects of treatment to prevent gout flares when starting allopurinol?". NIHR Evidence. 6 February 2024. doi:10.3310/nihrevidence_62005. S2CID 267539627. Archived from the original on 16 February 2024. Retrieved 16 February 2024.
- ^ a b "Information for Healthcare Professionals: New Safety Information for Colchicine (marketed as Colcrys)". U.S. Food and Drug Administration. Archived from the original on 18 October 2009.
- ^ McKenzie BJ, Wechalekar MD, Johnston RV, et al. (26 August 2021). "Colchicine for acute gout". The Cochrane Database of Systematic Reviews. 2021 (8): CD006190. doi:10.1002/14651858.CD006190.pub3. PMC 8407279. PMID 34438469.
- ^ Man CY, Cheung IT, Cameron PA, et al. (2007). "Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial" (PDF). Annals of Emergency Medicine. 49 (5): 670–677. doi:10.1016/j.annemergmed.2006.11.014. PMC 7115288. PMID 17276548. Archived (PDF) from the original on 20 October 2020. Retrieved 8 September 2019.
- ^ Wechalekar MD, Vinik O, Schlesinger N, et al. (30 April 2013). "Intra-articular glucocorticoids for acute gout". Cochrane Database of Systematic Reviews (4): CD009920. doi:10.1002/14651858.cd009920.pub2. ISSN 1465-1858. PMID 23633379.
- ^ Janssens HJ, Lucassen PL, Van de Laar FA, et al. (23 April 2008). "Systemic corticosteroids for acute gout" (PDF). Cochrane Database of Systematic Reviews. 2010 (2): CD005521. doi:10.1002/14651858.cd005521.pub2. hdl:2066/70896. ISSN 1465-1858. PMC 8276233. PMID 18425920. Archived from the original on 28 August 2021. Retrieved 24 September 2019.
- ^ a b c Sivera F, Wechalekar MD, Andrés M, et al. (2014). "Interleukin-1 inhibitors for acute gout". Cochrane Database of Systematic Reviews. 2014 (9): CD009993. doi:10.1002/14651858.CD009993.pub2. PMC 10891421. PMID 25177840.
- ^ a b Kim SY, De Vera MA, Choi HK (2008). "Gout and mortality". Clin. Exp. Rheumatol. 26 (5 Suppl 51): S115–S119. PMID 19026153.
- ^ Zhang T, Pope JE (30 March 2017). "Cardiovascular effects of urate-lowering therapies in patients with chronic gout: a systematic review and meta-analysis". Rheumatology. 56 (7): 1144–1153. doi:10.1093/rheumatology/kex065. PMID 28379501.
- ^ Vos T, Barber RM, Bell B, et al. (August 2015). "Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet. 386 (9995): 743–800. doi:10.1016/S0140-6736(15)60692-4. PMC 4561509. PMID 26063472.
- ^ Rheumatology Therapeutics Medical Center. "What Are the Risk Factors for Gout?". Archived from the original on 25 March 2007. Retrieved 26 January 2007.
- ^ Roberts-Thomson RA, Roberts-Thomson PJ (1 May 1999). "Rheumatic disease and the Australian Aborigine". Annals of the Rheumatic Diseases. 58 (5): 266–270. doi:10.1136/ard.58.5.266. PMC 1752880. PMID 10225809.
- ^ Fam AG (May 2000). "What is new about crystals other than monosodium urate?". Curr Opin Rheumatol. 12 (3): 228–234. doi:10.1097/00002281-200005000-00013. PMID 10803754.
- ^ Kuo CF, Grainge MJ, Zhang W, et al. (7 July 2015). "Global epidemiology of gout: prevalence, incidence and risk factors". Nature Reviews Rheumatology. 11 (11): 649–662. doi:10.1038/nrrheum.2015.91. ISSN 1759-4790. PMID 26150127.
- ^ a b c d Pillinger MH, Rosenthal P, Abeles AM (2007). "Hyperuricemia and gout: new insights into pathogenesis and treatment". Bulletin of the NYU Hospital for Joint Diseases. 65 (3): 215–221. PMID 17922673. Archived from the original on 16 December 2008.
- ^ Pierre-Jerome C (11 May 2022). "8.5: Gout and Charcot neuroarthropathy". The Essentials of Charcot Neuroarthropathy: Biomechanics, Pathophysiology, and MRI Findings. Amsterdam: Elsevier. p. 233. ISBN 9780323995788. Archived from the original on 28 April 2024. Retrieved 28 April 2024.
[...] Randolphus of Bocking [...] was the first to use the word 'gout' to express the clinical signs of podagra. Bocking was the domestic chaplain to Bishop of Chichester (1197-1258).
- ^ "gout, n.1". Oxford English Dictionary, Second edition, 1989. Archived from the original on 8 May 2020. Retrieved 18 September 2011.
- ^ "The Disease Of Kings". Forbes.com. Archived from the original on 1 September 2017.
It has been referred to, maybe a touch inaccurately, as 'The disease of kings and the king of diseases.'
- ^ Schwartz, Stephan A. "Disease of distinction." Explore 2, no. 6 (2006): 515–519. - "Both the Ebers and Edwin Smith Papyri describe a condition that is clearly gout.[...] They were written about 1552 BC but contain information taken from texts a thousand years earlier, and ascribed to Imhotep, a kind of ancient world Leonardo da Vinci, and the great overarching figure of Egyptian medicine."
- ^ "The Internet Classics Archive Aphorisms by Hippocrates". MIT. Archived from the original on 7 July 2010. Retrieved 27 July 2010.
- ^ Celsus AC. "On Medicine". University of Chicago. Book IV. Archived from the original on 10 July 2024. Retrieved 19 February 2021.
- ^ Copeman W (2021). A Short History of the Gout and the Rheumatic Diseases. University of California Press. p. 68. ISBN 978-0-520-33947-7.
- ^ "Gout – The Affliction of Kings". h2g2. BBC. 23 December 2012. Archived from the original on 11 September 2010.
- ^ Marryat T (1798) [1758]. Therapeutics: Or, the Art of Healing: To which is Added, a Glossary of the Most Difficult Words (14 ed.). Bristol: R. Edwards. p. 168. Archived from the original on 28 April 2024. Retrieved 28 April 2024.
- ^ Storey GD (October 2001). "Alfred Baring Garrod (1819–1907)". Rheumatology. 40 (10): 1189–1190. doi:10.1093/rheumatology/40.10.1189. PMID 11600751.
- ^ a b Agudelo CA, Wise CM (2001). "Gout: diagnosis, pathogenesis, and clinical manifestations". Curr Opin Rheumatol. 13 (3): 234–239. doi:10.1097/00002281-200105000-00015. PMID 11333355. S2CID 34502097.
- ^ Choi HK, Mount DB, Reginato AM, et al. (4 October 2005). "Pathogenesis of gout". Annals of Internal Medicine. 143 (7): 499–516. doi:10.7326/0003-4819-143-7-200510040-00009. PMID 16204163. S2CID 194570.
- ^ Rothschild BM, Tanke D, Carpenter K (1997). "Tyrannosaurs suffered from gout". Nature. 387 (6631): 357. Bibcode:1997Natur.387..357R. doi:10.1038/387357a0. PMID 9163417. S2CID 1360596. Archived from the original on 17 April 2021. Retrieved 29 September 2020.
- ^ Abeles, A. M., Pillinger, M. H. (8 March 2010). "New therapeutic options for gout here and on the horizon". Journal of Musculoskeletal Medicine. Archived from the original on 20 May 2010. Retrieved 23 April 2010.
- ^ Sivera F, Wechalekar MD, Andrés M, et al. (1 September 2014). "Interleukin-1 inhibitors for acute gout". The Cochrane Database of Systematic Reviews. 2014 (9): CD009993. doi:10.1002/14651858.CD009993.pub2. PMC 10891421. PMID 25177840.
External links
[edit]- Chisholm H, ed. (1911). . Encyclopædia Britannica. Vol. 12 (11th ed.). Cambridge University Press. pp. 289–291.
- "Gout". MedlinePlus. U.S. National Library of Medicine.


![Uric acid crystals in polarized light, showing negative birefringence, with yellow color when aligned parallel to the axis of the red compensator, and blue when aligned perpendicularly to it.[57]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b2/Birefringence_microscopy_of_gout%2C_annotated.jpg/200px-Birefringence_microscopy_of_gout%2C_annotated.jpg)