Triphenylmethyl radical

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Triphenylmethyl | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H15 | |
| Molar mass | 243.329 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The triphenylmethyl radical (often shorted to trityl radical) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trityl radical has been heavily exploited.[1]
Preparation and properties
[edit]The triphenylmethyl radical can be prepared by homolysis of triphenylmethyl chloride 1 by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid-type dimer 3 (Gomberg's dimer). In benzene the concentration of the radical is 2%.[2]
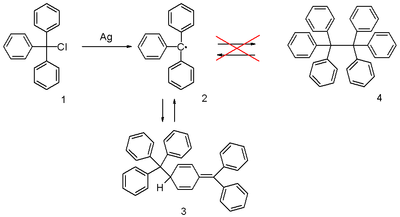
Solutions containing the radical are yellow; when the temperature of the solution is raised, the yellow color becomes more intense as the equilibrium is shifted in favor of the radical rather than the colorless dimer, in accordance with Le Chatelier's principle.
The triphenylmethyl radical exhibits green photoluminescence. Further reaction of the quinoid dimer with another triphenylmethyl radical produces a quinoid radical that exhibits red photoluminescence.[3]
When exposed to air, the radical rapidly oxidizes to the peroxide, and the color of the solution changes from yellow to colorless. Likewise, the radical reacts with iodine to triphenylmethyl iodide.
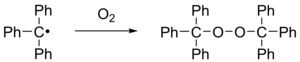
While the triphenyl radical itself forms a quinoid dimer, derivatives of the triphenyl radical with certain substituted phenyl groups do form dimers with a hexaphenylethane-like structure. For example, the tris(3,5-di-tert-butylphenyl) radical dimerizes to give hexakis(3,5-di-t-butylphenyl)ethane, with a bond length of 1.67 Å for the central carbon–carbon bond. Theoretical calculations on a very high level of theory indicate that van der Waals attraction between the tert-butyl groups create a potential minimum that is absent in the unsubstituted molecule.[4][5] Other derivatives have been reported as the quinoid dimer [6]
The class of triaryl-methyl radicals have applications in the synthesis of organic magnets.[7]
History
[edit]The radical was discovered by Moses Gomberg in 1900 at the University of Michigan.[8][9][10] He tried to prepare hexaphenylethane from triphenylmethyl chloride and zinc in benzene in a Wurtz reaction and found that the product, based on its behaviour towards iodine and oxygen, was far more reactive than anticipated. The discovered structure was used in the development of ESR spectroscopy and confirmed by it.[11][12][13] The triphenylmethyl radical, and the larger class of triarylmethyl radicals, are called Gomberg radicals.[3][7]
The correct quinoid structure for the dimer was suggested as early as 1904 but this structure was soon after abandoned by the scientific community in favor of hexaphenylethane (4).[14] It subsequently took until 1968 for its rediscovery when researchers at the Vrije Universiteit Amsterdam published proton NMR data.[15]
See also
[edit]References
[edit]- ^ Tidwell, Thomas T. (2010). "Triarylmethyl and Related Radicals". Stable Radicals. pp. 1–31. doi:10.1002/9780470666975.ch1. ISBN 9780470666975.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ a b Bulgakov, Ramil G.; Kuleshov, Sergei P.; Valiullina, Zemfira S. (2000). "Red photoluminescence in the synthesis of triphenylmethyl radicals by the Gomberg method". Mendeleev Communications. 10: 22–23. doi:10.1070/MC2000v010n01ABEH001115.
- ^ Lewars, Errol (2008), "8. Hexaphenylethane", Modeling Marvels, Springer, Bibcode:2008moma.book.....L
- ^ Grimme, Stefan; Schreiner, Peter R. (2011). "Steric crowding can stabilize a labile molecule: Solving the hexaphenylethane riddle". Angewandte Chemie International Edition. 50 (52): 12639–12642. doi:10.1002/anie.201103615. PMID 22025456.
- ^ Uchimura, Y.; Takeda, T.; Katoono, R.; Fujiwara, K.; Suzuki, T. (2015). "New Insights into the Hexaphenylethane Riddle: Formation of an α,o-Dimer". Angewandte Chemie International Edition. 54 (13): 4010–4013. doi:10.1002/anie.201500122. PMID 25704856.
- ^ a b Shishlov, Nikolay M. (2006). "From the Gomberg radical to organic magnets". Russian Chemical Reviews. 75 (10): 863–884. doi:10.1070/RC2006v075n10ABEH003621.
- ^ Gomberg, M. (1900). "An instance of trivalent carbon: triphenylmethyl". Journal of the American Chemical Society. 22 (11): 757–771. doi:10.1021/ja02049a006.
- ^ Gomberg, M. (1901). "On trivalent carbon". Journal of the American Chemical Society. 23 (7): 496–502. doi:10.1021/ja02033a015. (Note: radical is also called a cadicle.)
- ^ Gomberg, M. (1902). "On trivalent carbon". Journal of the American Chemical Society. 24 (7): 597–628. doi:10.1021/ja02021a001.
- ^ Weissman, S. I.; Sowden, John C. (1953). "Electron distribution in triphenylmethyl: Hyperfine structure of the paramagnetic resonance absorption of (C6H5)3C13*". Journal of the American Chemical Society. 75 (2): 503. doi:10.1021/ja01098a522.
- ^ Sinclair, J.; Kivelson, D. (1968). "Electron spin resonance studies of substituted triphenylmethyl radicals". Journal of the American Chemical Society. 90 (19): 5074–5080. doi:10.1021/ja01021a004.
- ^ "ESR spectrum of the triphenylmethyl radical". School of Chemistry, University of Bristol. Retrieved August 5, 2018.
- ^ McBride, J. M. (1974). "The hexaphenylethane riddle". Tetrahedron. 30 (14): 2009–2022. doi:10.1016/S0040-4020(01)97332-6.
- ^ Lankamp, H.; Nauta, W. Th.; MacLean, C. (1968). "A new interpretation of the monomer–dimer equilibrium of triphenylmethyl- and alkyl-substituted-diphenyl methyl-radicals in solution". Tetrahedron Letters. 9 (2): 249–254. doi:10.1016/S0040-4039(00)75598-5.
