Traumatic brain injury
| Traumatic brain injury | |
|---|---|
| Other names | Intracranial injury, physically induced brain injury[1] |
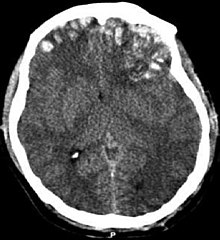 | |
| CT scan showing cerebral contusions, hemorrhage within the hemispheres, and subdural hematoma. There is also displaced skull fracture of left transverse parietal and temporal bones.[2] | |
| Specialty | Neurosurgery, pediatrics |
| Symptoms | Physical, cognitive, sensory, social, emotional, and behavioral symptoms |
| Types | Mild to severe[3] |
| Causes | Trauma to the head[3] |
| Risk factors | Old age,[3] alcohol |
| Diagnostic method | Based on neurological exam, medical imaging[4] |
| Treatment | Behavioral therapy, speech therapy |
A traumatic brain injury (TBI), also known as an intracranial injury, is an injury to the brain caused by an external force. TBI can be classified based on severity ranging from mild traumatic brain injury (mTBI/concussion) to severe traumatic brain injury.[5] TBI can also be characterized based on mechanism (closed or penetrating head injury) or other features (e.g., occurring in a specific location or over a widespread area).[6] Head injury is a broader category that may involve damage to other structures such as the scalp and skull. TBI can result in physical, cognitive, social, emotional and behavioral symptoms, and outcomes can range from complete recovery to permanent disability or death.
Causes include falls, vehicle collisions and violence. Brain trauma occurs as a consequence of a sudden acceleration or deceleration within the cranium or by a complex combination of both movement and sudden impact. In addition to the damage caused at the moment of injury, a variety of events following the injury may result in further injury. These processes may include alterations in cerebral blood flow and pressure within the skull. Some of the imaging techniques used for diagnosis of moderate to severe TBI include computed tomography (CT) and magnetic resonance imaging (MRIs).
Prevention measures include use of seat belts and helmets, not drinking and driving, fall prevention efforts in older adults and safety measures for children.[7] Depending on the injury, treatment required may be minimal or may include interventions such as medications, emergency surgery or surgery years later. Physical therapy, speech therapy, recreation therapy, occupational therapy and vision therapy may be employed for rehabilitation. Counseling, supported employment and community support services may also be useful.
TBI is a major cause of death and disability worldwide, especially in children and young adults.[8] Males sustain traumatic brain injuries around twice as often as females.[9] The 20th century saw developments in diagnosis and treatment that decreased death rates and improved outcomes.
Classification
Traumatic brain injury is defined as damage to the brain resulting from external mechanical force, such as rapid acceleration or deceleration, impact, blast waves, or penetration by a projectile.[10] Brain function is temporarily or permanently impaired and structural damage may or may not be detectable with current technology.[11]
TBI is one of two subsets of acquired brain injury (brain damage that occur after birth); the other subset is non-traumatic brain injury, which does not involve external mechanical force (examples include stroke and infection).[12][13] All traumatic brain injuries are head injuries, but the latter term may also refer to injury to other parts of the head;[14][15][16] however, the terms head injury and brain injury are often used interchangeably.[17] Similarly, brain injuries fall under the classification of central nervous system injuries[18] and neurotrauma.[19] In neuropsychology research literature, in general the term "traumatic brain injury" is used to refer to non-penetrating traumatic brain injuries.
TBI is usually classified based on severity, anatomical features of the injury, and the mechanism (the causative forces).[20] Mechanism-related classification divides TBI into closed and penetrating head injury.[10] A closed (also called nonpenetrating, or blunt)[14] injury occurs when the brain is not exposed.[15] A penetrating, or open, head injury occurs when an object pierces the skull and breaches the dura mater, the outermost membrane surrounding the brain.[15]
Severity
| GCS | PTA | LOC | |
|---|---|---|---|
| Mild | 13–15 | <1 day |
0–30 minutes |
| Moderate | 9–12 | >1 to <7 days |
>30 min to <24 hours |
| Severe | 3–8 | >7 days | >24 hours |
Brain injuries can be classified into mild, moderate, and severe categories.[20][5] The Glasgow Coma Scale (GCS), the most commonly used system for classifying TBI severity, grades a person's level of consciousness on a scale of 3–15 based on verbal, motor, and eye-opening reactions to stimuli.[22] In general, it is agreed that a TBI with a GCS of 13 or above is mild, 9–12 is moderate, and 8 or below is severe.[11][16][23] Similar systems exist for young children;[16] however, the GCS grading system has limited ability to predict outcomes. Because of this, other classification systems such as the one shown in the table are also used to help determine severity. A current model developed by the Department of Defense and Department of Veterans Affairs uses all three criteria of GCS after resuscitation, duration of post-traumatic amnesia (PTA), and loss of consciousness (LOC).[21] It also has been proposed to use changes that are visible on neuroimaging, such as swelling, focal lesions, or diffuse injury as method of classification.[10]
Pathological features
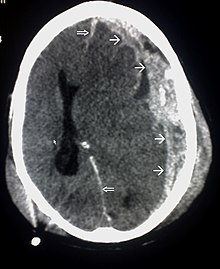
Systems also exist to classify TBI by its pathological features.[20] Lesions can be extra-axial, (occurring within the skull but outside of the brain) or intra-axial (occurring within the brain tissue).[24] Damage from TBI can be focal or diffuse, confined to specific areas or distributed in a more general manner, respectively;[25] however, it is common for both types of injury to exist in a given case.[25]
Diffuse injury manifests with little apparent damage in neuroimaging studies, but lesions can be seen with microscopy techniques post-mortem,[25][26] and in the early 2000s, researchers discovered that diffusion tensor imaging (DTI), a way of processing MRI images that shows white matter tracts, was an effective tool for displaying the extent of diffuse axonal injury.[27][28] Types of injuries considered diffuse include edema (swelling), concussion and diffuse axonal injury, which is widespread damage to axons including white matter tracts and projections to the cortex.[29][30]
Focal injuries often produce symptoms related to the functions of the damaged area.[18] Research shows that the most common areas to have focal lesions in non-penetrating traumatic brain injury are the orbitofrontal cortex (the lower surface of the frontal lobes) and the anterior temporal lobes, areas that are involved in social behavior, emotion regulation, olfaction, and decision-making, hence the common social/emotional and judgment deficits following moderate-severe TBI.[31][32][33][34] Symptoms such as hemiparesis or aphasia can also occur when less commonly affected areas such as motor or language areas are, respectively, damaged.[35][36]
One type of focal injury, cerebral laceration, occurs when the tissue is cut or torn.[37] Such tearing is common in orbitofrontal cortex in particular, because of bony protrusions on the interior skull ridge above the eyes.[31] In a similar injury, cerebral contusion (bruising of brain tissue), blood is mixed among tissue.[23] In contrast, intracranial hemorrhage involves bleeding that is not mixed with tissue.[37]
Hematomas, also focal lesions, are collections of blood in or around the brain that can result from hemorrhage.[11] Intracerebral hemorrhage, with bleeding in the brain tissue itself, is an intra-axial lesion. Extra-axial lesions include epidural hematoma, subdural hematoma, subarachnoid hemorrhage, and intraventricular hemorrhage.[38] Epidural hematoma involves bleeding into the area between the skull and the dura mater, the outermost of the three membranes surrounding the brain.[11] In subdural hematoma, bleeding occurs between the dura and the arachnoid mater.[23] Subarachnoid hemorrhage involves bleeding into the space between the arachnoid membrane and the pia mater.[23] Intraventricular hemorrhage occurs when there is bleeding in the ventricles.[38]
Signs and symptoms

Symptoms are dependent on the type of TBI (diffuse or focal) and the part of the brain that is affected.[40] Unconsciousness tends to last longer for people with injuries on the left side of the brain than for those with injuries on the right.[15] Symptoms are also dependent on the injury's severity. With mild TBI, the patient may remain conscious or may lose consciousness for a few seconds or minutes.[41] Other symptoms of mild TBI include headache, vomiting, nausea, lack of motor coordination, dizziness, difficulty balancing,[42] lightheadedness, blurred vision or tired eyes, ringing in the ears, bad taste in the mouth, fatigue or lethargy, and changes in sleep patterns.[41] Cognitive and emotional symptoms include behavioral or mood changes, confusion, and trouble with memory, concentration, attention, or thinking.[41] Mild TBI symptoms may also be present in moderate and severe injuries.[41]
A person with a moderate or severe TBI may have a headache that does not go away, repeated vomiting or nausea, convulsions, an inability to awaken, dilation of one or both pupils, slurred speech, aphasia (word-finding difficulties), dysarthria (muscle weakness that causes disordered speech), weakness or numbness in the limbs, loss of coordination, confusion, restlessness, or agitation.[41] Common long-term symptoms of moderate to severe TBI are changes in appropriate social behavior, deficits in social judgment, and cognitive changes, especially problems with sustained attention, processing speed, and executive functioning.[34][43][44][45][46] Alexithymia, a deficiency in identifying, understanding, processing, and describing emotions occurs in 60.9% of individuals with TBI.[47] Cognitive and social deficits have long-term consequences for the daily lives of people with moderate to severe TBI, but can be improved with appropriate rehabilitation.[46][48][49][50]
When the pressure within the skull (intracranial pressure, abbreviated ICP) rises too high, it can be deadly.[51] Signs of increased ICP include decreasing level of consciousness, paralysis or weakness on one side of the body, and a blown pupil, one that fails to constrict in response to light or is slow to do so.[51] Cushing's triad, a slow heart rate with high blood pressure and respiratory depression is a classic manifestation of significantly raised ICP.[11] Anisocoria, unequal pupil size, is another sign of serious TBI.[39] Abnormal posturing, a characteristic positioning of the limbs caused by severe diffuse injury or high ICP, is an ominous sign.[11]
Small children with moderate to severe TBI may have some of these symptoms but have difficulty communicating them.[52] Other signs seen in young children include persistent crying, inability to be consoled, listlessness, refusal to nurse or eat,[52] and irritability.[11]
Causes
The most common causes of TBI in the U.S. include violence, transportation accidents, construction site mishaps, and sports.[42][53] Motor bikes are major causes, increasing in significance in developing countries as other causes reduce.[54][55] The estimates that between 1.6 and 3.8 million traumatic brain injuries each year are a result of sports and recreation activities in the US.[56] In children aged two to four, falls are the most common cause of TBI, while in older children traffic accidents compete with falls for this position.[57] TBI is the third most common injury to result from child abuse.[58] Abuse causes 19% of cases of pediatric brain trauma, and the death rate is higher among these cases.[59] Although men are twice as likely to have a TBI. Domestic violence is another cause of TBI,[60] as are work-related and industrial accidents.[61] Firearms[15] and blast injuries from explosions[62] are other causes of TBI, which is the leading cause of death and disability in war zones.[63] According to Representative Bill Pascrell (Democrat, NJ), TBI is "the signature injury of the wars in Iraq and Afghanistan."[64] There is a promising technology called activation database-guided EEG biofeedback, which has been documented to return a TBI's auditory memory ability to above the control group's performance[65][66]
Mechanism
Physical forces

The type, direction, intensity, and duration of forces all contribute to the characteristics and severity of TBI.[10] Forces that may contribute to TBI include angular, rotational, shear, and translational forces.[37]
Even in the absence of an impact, significant acceleration or deceleration of the head can cause TBI; however in most cases, a combination of impact and acceleration is probably to blame.[37] Forces involving the head striking or being struck by something, termed contact or impact loading, are the cause of most focal injuries, and movement of the brain within the skull, termed noncontact or inertial loading, usually causes diffuse injuries.[20] The violent shaking of an infant that causes shaken baby syndrome commonly manifests as diffuse injury.[68] In impact loading, the force sends shock waves through the skull and brain, resulting in tissue damage.[37] Shock waves caused by penetrating injuries can also destroy tissue along the path of a projectile, compounding the damage caused by the missile itself.[23]
Damage may occur directly under the site of impact, or it may occur on the side opposite the impact (coup and contrecoup injury, respectively).[67] When a moving object impacts the stationary head, coup injuries are typical,[69] while contrecoup injuries are usually produced when the moving head strikes a stationary object.[70]
Primary and secondary injury

A large percentage of the people killed by brain trauma do not die right away but rather days to weeks after the event;[71] rather than improving after being hospitalized, some 40% of TBI patients deteriorate.[72] Primary brain injury (the damage that occurs at the moment of trauma when tissues and blood vessels are stretched, compressed, and torn) is not adequate to explain this deterioration; rather, it is caused by secondary injury, a complex set of cellular processes and biochemical cascades that occur in the minutes to days following the trauma.[73] These secondary processes can dramatically worsen the damage caused by primary injury[63] and account for the greatest number of TBI deaths occurring in hospitals.[39]
Secondary injury events include damage to the blood–brain barrier, release of factors that cause inflammation, free radical overload, excessive release of the neurotransmitter glutamate (excitotoxicity), influx of calcium and sodium ions into neurons, and dysfunction of mitochondria.[63] Injured axons in the brain's white matter may separate from their cell bodies as a result of secondary injury,[63] potentially killing those neurons. Other factors in secondary injury are changes in the blood flow to the brain; ischemia (insufficient blood flow); cerebral hypoxia (insufficient oxygen in the brain); cerebral edema (swelling of the brain); and raised intracranial pressure (the pressure within the skull).[74] Intracranial pressure may rise due to swelling or a mass effect from a lesion, such as a hemorrhage.[51] As a result, cerebral perfusion pressure (the pressure of blood flow in the brain) is reduced; ischemia results.[39][75] When the pressure within the skull rises too high, it can cause brain death or brain herniation, in which parts of the brain are squeezed by structures in the skull.[51]
Diagnosis

Diagnosis is suspected based on lesion circumstances and clinical evidence, most prominently a neurological examination, for example checking whether the pupils constrict normally in response to light and assigning a Glasgow Coma Score.[23] Neuroimaging helps in determining the diagnosis and prognosis and in deciding what treatments to give.[76] DSM-5 can be utilized to diagnose TBI and its psychiatric sequelae.[77][78][79]
The preferred radiologic test in the emergency setting is computed tomography (CT): it is quick, accurate, and widely available.[80] Follow-up CT scans may be performed later to determine whether the injury has progressed.[10]
Magnetic resonance imaging (MRI) can show more detail than CT, and can add information about expected outcome in the long term.[23] It is more useful than CT for detecting injury characteristics such as diffuse axonal injury in the longer term;[10] however, MRI is not used in the emergency setting for reasons including its relative inefficacy in detecting bleeds and fractures, its lengthy acquisition of images, the inaccessibility of the patient in the machine, and its incompatibility with metal items used in emergency care.[23] A variant of MRI since 2012 is high-definition fiber tracking (HDFT).[81]
Other techniques may be used to confirm a particular diagnosis. X-rays are still used for head trauma, but evidence suggests they are not useful; head injuries are either so mild that they do not need imaging or severe enough to merit the more accurate CT.[80] Angiography may be used to detect blood vessel pathology when risk factors such as penetrating head trauma are involved.[10] Functional imaging can measure cerebral blood flow or metabolism, inferring neuronal activity in specific regions and potentially helping to predict outcome.[82]
Neuropsychological assessment can be performed to evaluate the long-term cognitive sequelae and to aid in the planning of the rehabilitation.[76] Instruments range from short measures of general mental functioning to complete batteries formed of different domain-specific tests.[83][84]
Prevention


Since a major cause of TBI are vehicle accidents, their prevention or the amelioration of their consequences can both reduce the incidence and gravity of TBI. In accidents, damage can be reduced by use of seat belts, child safety seats[56] and motorcycle helmets,[86] and presence of roll bars and airbags.[37] Education programs exist to lower the number of crashes.[76] In addition, changes to public policy and safety laws can be made; these include speed limits, seat belt and helmet laws, and road engineering practices.[63]
Changes to common practices in sports have also been discussed. An increase in use of helmets could reduce the incidence of TBI.[63] Due to the possibility that repeatedly "heading" a ball practicing soccer could cause cumulative brain injury, the idea of introducing protective headgear for players has been proposed.[87] Improved equipment design can enhance safety; softer baseballs reduce head injury risk.[88] Rules against dangerous types of contact, such as "spear tackling" in American football, when one player tackles another head first, may also reduce head injury rates.[88]
Falls can be avoided by installing grab bars in bathrooms and handrails on stairways; removing tripping hazards such as throw rugs; or installing window guards and safety gates at the top and bottom of stairs around young children.[56] Playgrounds with shock-absorbing surfaces such as mulch or sand also prevent head injuries.[56] Child abuse prevention is another tactic; programs exist to prevent shaken baby syndrome by educating about the dangers of shaking children.[59] Gun safety, including keeping guns unloaded and locked, is another preventative measure.[89] Studies on the effect of laws that aim to control access to guns in the United States have been insufficient to determine their effectiveness preventing number of deaths or injuries.[90]
Treatment
It is important to begin emergency treatment within the so-called "golden hour" following the injury.[91] People with moderate to severe injuries are likely to receive treatment in an intensive care unit followed by a neurosurgical ward.[92] Treatment depends on the recovery stage of the patient. In the acute stage, the primary aim is to stabilize the patient and focus on preventing further injury. This is done because the initial damage caused by trauma cannot be reversed.[92] Rehabilitation is the main treatment for the subacute and chronic stages of recovery.[92] International clinical guidelines have been proposed with the aim of guiding decisions in TBI treatment, as defined by an authoritative examination of current evidence.[10]
Acute stage
Tranexamic acid within three hours of a head injury decreases the risk of death.[93] Certain facilities are equipped to handle TBI better than others; initial measures include transporting patients to an appropriate treatment center.[51][94] Both during transport and in hospital the primary concerns are ensuring proper oxygen supply, maintaining adequate blood flow to the brain, and controlling raised intracranial pressure (ICP),[11] since high ICP deprives the brain of badly needed blood flow[95] and can cause deadly brain herniation. Other methods to prevent damage include management of other injuries and prevention of seizures.[23][76] Some data supports the use of hyperbaric oxygen therapy to improve outcomes.[96] Further research is required to determine the effectiveness and clinical importance of positioning the head at different angles (degrees of head-of-bed elevation) while the person is being treated in intensive care.[97]
Neuroimaging is helpful but not flawless in detecting raised ICP.[98] A more accurate way to measure ICP is to place a catheter into a ventricle of the brain,[39] which has the added benefit of allowing cerebrospinal fluid to drain, releasing pressure in the skull.[39] Treatment of raised ICP may be as simple as tilting the person's bed and straightening the head to promote blood flow through the veins of the neck. Sedatives, analgesics and paralytic agents are often used.[51] Propofol and midazolam are equally effective as sedatives.[99]
Hypertonic saline can improve ICP by reducing the amount of cerebral water (swelling), though it is used with caution to avoid electrolyte imbalances or heart failure.[10][100][101] Mannitol, an osmotic diuretic,[10] appears to be as effective as hypertonic saline at reducing ICP;[102][103][100][104] however, some concerns have been raised regarding some of the studies performed.[105][specify] Hyertonic saline is also suitable in children with severe traumatic brain injury.[106]
Diuretics, drugs that increase urine output to reduce excessive fluid in the system, may be used to treat high intracranial pressures, but may cause hypovolemia (insufficient blood volume).[39] Hyperventilation (larger and/or faster breaths) reduces carbon dioxide levels and causes blood vessels to constrict; this decreases blood flow to the brain and reduces ICP,[107] but it potentially causes ischemia[11][39][108] and is, therefore, used only in the short term.[11] Giving corticosteroids is associated with an increased risk of death, and so their routine use is not recommended.[109][110] There is no strong evidence that the following pharmaceutical interventions should be recommended to routinely treat TBI: magnesium, monoaminergic and dopamine agonists, progesterone, aminosteroids, excitatory amino acid reuptake inhibitors, beta-2 antagonists (bronchodilators), haemostatic and antifibrinolytic drugs.[99][111][112][113][114]
Endotracheal intubation and mechanical ventilation may be used to ensure proper oxygen supply and provide a secure airway.[76] Hypotension (low blood pressure), which has a devastating outcome in TBI, can be prevented by giving intravenous fluids to maintain a normal blood pressure. Failing to maintain blood pressure can result in inadequate blood flow to the brain.[23] Blood pressure may be kept at an artificially high level under controlled conditions by infusion of norepinephrine or similar drugs; this helps maintain cerebral perfusion.[115] Body temperature is carefully regulated because increased temperature raises the brain's metabolic needs, potentially depriving it of nutrients.[116] Seizures are common. While they can be treated with benzodiazepines, these drugs are used carefully because they can depress breathing and lower blood pressure.[51] Anti-convulsant medications have only been found to be useful for reducing the risk of an early seizure.[99] Phenytoin and Levetiracetam appear to have similar levels of effectiveness for preventing early seizures.[99] People with TBI are more susceptible to side effects and may react adversely to some medications.[92] During treatment monitoring continues for signs of deterioration such as a decreasing level of consciousness.[10][11]
Traumatic brain injury may cause a range of serious coincidental complications that include cardiac arrhythmias[117] and neurogenic pulmonary edema.[118] These conditions must be adequately treated and stabilised as part of the core care. Surgery can be performed on mass lesions or to eliminate objects that have penetrated the brain. Mass lesions such as contusions or hematomas causing a significant mass effect (shift of intracranial structures) are considered emergencies and are removed surgically.[23] For intracranial hematomas, the collected blood may be removed using suction or forceps or it may be floated off with water.[23] Surgeons look for hemorrhaging blood vessels and seek to control bleeding.[23] In penetrating brain injury, damaged tissue is surgically debrided, and craniotomy may be needed.[23] Craniotomy, in which part of the skull is removed, may be needed to remove pieces of fractured skull or objects embedded in the brain.[119] Decompressive craniectomy (DC) is performed routinely in the very short period following TBI during operations to treat hematomas; part of the skull is removed temporarily (primary DC).[120] DC performed hours or days after TBI in order to control persistently high intracranial pressures (secondary DC), although can reduce intracranial pressure and length of stay in ICU, but have worse Glasgow Coma Scale (GCS) scores, and high chances of death, vegetative state, or severe disability when compared to those receiving standard medical therapies.[121][122][10][120]
Chronic stage
Once medically stable, people may be transferred to a subacute rehabilitation unit of the medical center or to an independent rehabilitation hospital.[92] Rehabilitation aims to improve independent functioning at home and in society, and to help adapt to disabilities.[92] Rehabilitation has demonstrated its general effectiveness when conducted by a team of health professionals who specialize in head trauma.[123] As for any person with neurologic deficits, a multidisciplinary approach is key to optimizing outcome. Physiatrists or neurologists are likely to be the key medical staff involved, but depending on the person, doctors of other medical specialties may also be helpful. Allied health professions such as physiotherapy, speech and language therapy, cognitive rehabilitation therapy, and occupational therapy will be essential to assess function and design the rehabilitation activities for each person.[124] Treatment of neuropsychiatric symptoms such as emotional distress and clinical depression may involve mental health professionals such as therapists, psychologists, and psychiatrists, while neuropsychologists can help to evaluate and manage cognitive deficits.[92][125] Social workers, rehabilitation support personnel, nutritionists, therapeutic recreationists, and pharmacists are also important members of the TBI rehabilitation team.[124] After discharge from the inpatient rehabilitation treatment unit, care may be given on an outpatient basis. Community-based rehabilitation will be required for a high proportion of people, including vocational rehabilitation; this supportive employment matches job demands to the worker's abilities.[126] People with TBI who cannot live independently or with family may require care in supported living facilities such as group homes.[126] Respite care, including day centers and leisure facilities for disabled people, offers time off for caregivers, and activities for people with TBI.[126]
Pharmacological treatment can help to manage psychiatric or behavioral problems.[127] Medication is also used to control post-traumatic epilepsy; however the preventive use of anti-epileptics is not recommended.[128] In those cases where the person is bedridden due to a reduction of consciousness, has to remain in a wheelchair because of mobility problems, or has any other problem heavily impacting self-caring capacities, caregiving and nursing are critical. The most effective research documented intervention approach is the activation database guided EEG biofeedback approach, which has shown significant improvements in memory abilities of the TBI subject that are far superior than traditional approaches (strategies, computers, medication intervention). Gains of 2.61 standard deviations have been documented. The TBI's auditory memory ability was superior to the control group after the treatment.[65]
Effect on the gait pattern

In patients who have developed paralysis of the legs in the form of spastic hemiplegia or diplegia as a result of the traumatic brain injury, various gait patterns can be observed, the exact extent of which can only be described with the help of complex gait analysis systems. In order to facilitate interdisciplinary communication in the interdisciplinary team between those affected, doctors, physiotherapists and orthotists, a simple description of the gait pattern is useful. J. Rodda and H. K. Graham already described in 2001 how gait patterns of CP patients can be more easily recognized and defined gait types which they compared in a classification. They also described that gait patterns can vary with age.[129] Building on this, the Amsterdam Gait Classification was developed at the free university in Amsterdam, the VU medisch centrum. A special feature of this classification is that it makes different gait patterns very recognizable and can be used in patients in whom only one leg and both legs are affected. The Amsterdam Gait Classification was developed for viewing patients with cerebral palsy; however, it can be used just as well in patients with traumatic brain injuries. According to the Amsterdam Gait Classification, five gait types are described. To assess the gait pattern, the patient is viewed visually or via a video recording from the side of the leg to be assessed. At the point in time at which the leg to be viewed is in mid stance and the leg not to be viewed is in mid swing, the knee angle and the contact of the foot with the ground are assessed on the one hand.[130]
Classification of the gait pattern according to the Amsterdam Gait Classification: In gait type 1, the knee angle is normal and the foot contact is complete. In gait type 2, the knee angle is hyperextended and the foot contact is complete. In gait type 3, the knee angle is hyperextended and foot contact is incomplete (only on the forefoot). In gait type 4, the knee angle is bent and foot contact is incomplete (only on the forefoot). With gait type 5, which is also known as crouch gait, the knee angle is bent and the foot contact is complete.[130]
Orthotics

To improve the gait pattern, orthotics can be included in the therapy concept.[131] An Orthosis can support physiotherapeutic treatment in setting the right motor impulses in order to create new cerebral connections.[132] The orthosis must meet the requirements of the medical prescription. In addition, the orthosis must be designed by the orthotist in such a way that it achieves the effectiveness of the necessary levers, matching the gait pattern, in order to support the proprioceptive approaches of physiotherapy. The orthotic concepts of the treatment are based on the concepts for the patients with cerebral palsy. The characteristics of the stiffness of the orthosis shells and the adjustable dynamics in the ankle joint are important elements of the orthosis to be considered.[133]
The orthotic concepts of the treatment are based on the concepts for the patients with cerebral palsy. Due to these requirements, the development of orthoses has changed significantly in recent years, especially since around 2010. At about the same time, care concepts were developed that deal intensively with the orthotic treatment of the lower extremities in cerebral palsy.[134] Modern materials and new functional elements enable the rigidity to be specifically adapted to the requirements that fits to the gait pattern of the patient.[135] The adjustment of the stiffness has a decisive influence on the gait pattern and on the energy cost of walking.[136][137][138] It is of great advantage if the stiffness of the orthosis can be adjusted separately from one another via resistances of the two functional elements in the two directions of movement, dorsiflexion and plantar flexion.[139]
Prognosis
Prognosis worsens with the severity of injury.[9] Most TBIs are mild and do not cause permanent or long-term disability; however, all severity levels of TBI have the potential to cause significant, long-lasting disability.[140] Permanent disability is thought to occur in 10% of mild injuries, 66% of moderate injuries, and 100% of severe injuries.[141] Most mild TBI is completely resolved within three weeks. Almost all people with mild TBI are able to live independently and return to the jobs they had before the injury, although a small portion have mild cognitive and social impairments.[89] Over 90% of people with moderate TBI are able to live independently, although some require assistance in areas such as physical abilities, employment, and financial managing.[89] Most people with severe closed head injury either die or recover enough to live independently; middle ground is less common.[10] Coma, as it is closely related to severity, is a strong predictor of poor outcome.[11]
Prognosis differs depending on the severity and location of the lesion, and access to immediate, specialised acute management. Subarachnoid hemorrhage approximately doubles mortality.[142] Subdural hematoma is associated with worse outcome and increased mortality, while people with epidural hematoma are expected to have a good outcome if they receive surgery quickly.[76] Diffuse axonal injury may be associated with coma when severe, and poor outcome.[10] Following the acute stage, prognosis is strongly influenced by the patient's involvement in activity that promote recovery, which for most patients requires access to a specialised, intensive rehabilitation service. The Functional Independence Measure is a way to track progress and degree of independence throughout rehabilitation.[143]
Medical complications are associated with a bad prognosis. Examples of such complications include: hypotension (low blood pressure), hypoxia (low blood oxygen saturation), lower cerebral perfusion pressures, and longer times spent with high intracranial pressures.[10][76] Patient characteristics also influence prognosis. Examples of factors thought to worsen it include: abuse of substances such as illicit drugs and alcohol and age over sixty or under two years (in children, younger age at time of injury may be associated with a slower recovery of some abilities).[76] Other influences that may affect recovery include pre-injury intellectual ability, coping strategies, personality traits, family environment, social support systems and financial circumstances.[144]
Life satisfaction has been known to decrease for individuals with TBI immediately following the trauma, but evidence has shown that life roles, age, and depressive symptoms influence the trajectory of life satisfaction as time passes.[145] Many people with traumatic brain injuries have poor physical fitness following their acute injury and this may result with difficulties in day-to-day activities and increased levels of fatigue.[146]
Complications

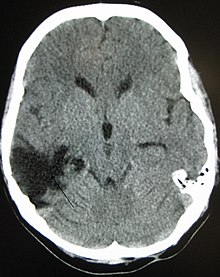
Improvement of neurological function usually occurs for two or more years after the trauma. For many years it was believed that recovery was fastest during the first six months, but there is no evidence to support this. It may be related to services commonly being withdrawn after this period, rather than any physiological limitation to further progress.[10] Children recover better in the immediate time frame and improve for longer periods.[11]
Complications are distinct medical problems that may arise as a result of the TBI. The results of traumatic brain injury vary widely in type and duration; they include physical, cognitive, emotional, and behavioral complications. TBI can cause prolonged or permanent effects on consciousness, such as coma, brain death, persistent vegetative state (in which patients are unable to achieve a state of alertness to interact with their surroundings),[147] and minimally conscious state (in which patients show minimal signs of being aware of self or environment).[148][149] Lying still for long periods can cause complications including pressure sores, pneumonia or other infections, progressive multiple organ failure,[92] and deep venous thrombosis, which can cause pulmonary embolism.[23] Infections that can follow skull fractures and penetrating injuries include meningitis and abscesses.[92] Complications involving the blood vessels include vasospasm, in which vessels constrict and restrict blood flow, the formation of aneurysms, in which the side of a vessel weakens and balloons out, and stroke.[92]
Movement disorders that may develop after TBI include tremor, ataxia (uncoordinated muscle movements), spasticity (muscle contractions are overactive), myoclonus (shock-like contractions of muscles), and loss of movement range and control (in particular with a loss of movement repertoire).[92][150] The risk of post-traumatic seizures increases with severity of trauma and is particularly elevated with certain types of brain trauma such as cerebral contusions or hematomas.[141] People with early seizures, those occurring within a week of injury, have an increased risk of post-traumatic epilepsy (recurrent seizures occurring more than a week after the initial trauma).[151] People may lose or experience altered vision, hearing, or smell.[11]
Hormonal disturbances may occur secondary to hypopituitarism, occurring immediately or years after injury in 10 to 15% of TBI patients. Development of diabetes insipidus or an electrolyte abnormality acutely after injury indicate need for endocrinologic work up. Signs and symptoms of hypopituitarism may develop and be screened for in adults with moderate TBI and in mild TBI with imaging abnormalities. Children with moderate to severe head injury may also develop hypopituitarism. Screening should take place 3 to 6 months, and 12 months after injury, but problems may occur more remotely.[152]
Cognitive deficits that can follow TBI include impaired attention; disrupted insight, judgement, and thought; reduced processing speed; distractibility; and deficits in executive functions such as abstract reasoning, planning, problem-solving, and multitasking.[153] Memory loss, the most common cognitive impairment among head-injured people, occurs in 20–79% of people with closed head trauma, depending on severity.[154] People who have had TBI may also have difficulty with understanding or producing spoken or written language, or with more subtle aspects of communication such as body language.[92] Post-concussion syndrome, a set of lasting symptoms experienced after mild TBI, can include physical, cognitive, emotional and behavioral problems such as headaches, dizziness, difficulty concentrating, and depression.[11] Multiple TBIs may have a cumulative effect.[149] A young person who receives a second concussion before symptoms from another one have healed may be at risk for developing a very rare but deadly condition called second-impact syndrome, in which the brain swells catastrophically after even a mild blow, with debilitating or deadly results. About one in five career boxers is affected by chronic traumatic brain injury (CTBI), which causes cognitive, behavioral, and physical impairments.[155] Dementia pugilistica, the severe form of CTBI, affects primarily career boxers years after a boxing career. It commonly manifests as dementia, memory problems, and parkinsonism (tremors and lack of coordination).[156]
TBI may cause emotional, social, or behavioral problems and changes in personality.[157][158][159][160] These may include emotional instability, depression, anxiety, hypomania, mania, apathy, irritability, problems with social judgment, and impaired conversational skills.[157][160][161][162] TBI appears to predispose survivors to psychiatric disorders including obsessive compulsive disorder, substance abuse, dysthymia, clinical depression, bipolar disorder, and anxiety disorders.[163] In patients who have depression after TBI, suicidal ideation is not uncommon; the suicide rate among these persons is increased 2- to 3-fold.[164] Social and behavioral symptoms that can follow TBI include disinhibition, inability to control anger, impulsiveness, lack of initiative, inappropriate sexual activity, asociality and social withdrawal, and changes in personality.[157][159][160][165]
TBI also has a substantial impact on the functioning of family systems[166] Caregiving family members and TBI survivors often significantly alter their familial roles and responsibilities following injury, creating significant change and strain on a family system. Typical challenges identified by families recovering from TBI include: frustration and impatience with one another, loss of former lives and relationships, difficulty setting reasonable goals, inability to effectively solve problems as a family, increased level of stress and household tension, changes in emotional dynamics, and overwhelming desire to return to pre-injury status. In addition, families may exhibit less effective functioning in areas including coping, problem solving and communication. Psychoeducation and counseling models have been demonstrated to be effective in minimizing family disruption.[167]
Epidemiology

TBI is a leading cause of death and disability around the globe[8] and presents a major worldwide social, economic, and health problem.[10] It is the number one cause of coma,[169] it plays the leading role in disability due to trauma,[76] and is the leading cause of brain damage in children and young adults.[15] In Europe it is responsible for more years of disability than any other cause.[10] It also plays a significant role in half of trauma deaths.[23]
Findings on the frequency of each level of severity vary based on the definitions and methods used in studies. A World Health Organization study estimated that between 70 and 90% of head injuries that receive treatment are mild,[170] and a US study found that moderate and severe injuries each account for 10% of TBIs, with the rest mild.[72]
The incidence of TBI varies by age, gender, region and other factors.[171] Findings of incidence and prevalence in epidemiological studies vary based on such factors as which grades of severity are included, whether deaths are included, whether the study is restricted to hospitalized people, and the study's location.[15] The annual incidence of mild TBI is difficult to determine but may be 100–600 people per 100,000.[63]
Mortality
In the US, the case fatality rate is estimated to be 21% by 30 days after TBI.[94] A study on Iraq War soldiers found that severe TBI carries a mortality of 30–50%.[63] Deaths have declined due to improved treatments and systems for managing trauma in societies wealthy enough to provide modern emergency and neurosurgical services.[116] The fraction of those who die after being hospitalized with TBI fell from almost half in the 1970s to about a quarter at the beginning of the 21st century.[76] This decline in mortality has led to a concomitant increase in the number of people living with disabilities that result from TBI.[172]
Biological, clinical, and demographic factors contribute to the likelihood that an injury will be fatal.[168] In addition, outcome depends heavily on the cause of head injury. In the US, patients with fall-related TBIs have an 89% survival rate, while only 9% of patients with firearm-related TBIs survive.[173] In the US, firearms are the most common cause of fatal TBI, followed by vehicle accidents and then falls.[168] Of deaths from firearms, 75% are considered to be suicides.[168]
The incidence of TBI is increasing globally, due largely to an increase in motor vehicle use in low- and middle-income countries.[10] In developing countries, automobile use has increased faster than safety infrastructure could be introduced.[63] In contrast, vehicle safety laws have decreased rates of TBI in high-income countries,[10] which have seen decreases in traffic-related TBI since the 1970s.[54] Each year in the United States, about two million people have a TBI,[21] approximately 675,000 injuries are seen in the emergency department,[174] and about 500,000 patients are hospitalized.[171] The yearly incidence of TBI is estimated at 180–250 per 100,000 people in the US,[171] 281 per 100,000 in France, 361 per 100,000 in South Africa, 322 per 100,000 in Australia,[15] and 430 per 100,000 in England.[61] In the European Union the yearly aggregate incidence of TBI hospitalizations and fatalities is estimated at 235 per 100,000.[10]
Demographics
TBI is present in 85% of traumatically injured children, either alone or with other injuries.[175] The greatest number of TBIs occur in people aged 15–24.[13][37] Because TBI is more common in young people, its costs to society are high due to the loss of productive years to death and disability.[10] The age groups most at risk for TBI are children ages five to nine and adults over age 80,[9] and the highest rates of death and hospitalization due to TBI are in people over age 65.[140] The incidence of fall-related TBI in First-World countries is increasing as the population ages; thus the median age of people with head injuries has increased.[10]
Regardless of age, TBI rates are higher in males.[37] Men have twice as many TBIs as women do and have a fourfold risk of fatal head injury,[9] and males account for two thirds of childhood and adolescent head trauma;[176] however, when matched for severity of injury, women appear to fare more poorly than men.[95]
Socioeconomic status also appears to affect TBI rates; people with lower levels of education and employment and lower socioeconomic status are at greater risk.[15] Approximately half of those incarcerated in prisons and jails in the United States have had TBIs.[177]
History

Head injury is present in ancient myths that may date back before recorded history.[178] Skulls found in battleground graves with holes drilled over fracture lines suggest that trepanation may have been used to treat TBI in ancient times.[179] Ancient Mesopotamians knew of head injury and some of its effects, including seizures, paralysis, and loss of sight, hearing or speech.[180] The Edwin Smith Papyrus, written around 1650–1550 BC, describes various head injuries and symptoms and classifies them based on their presentation and tractability.[181] Ancient Greek physicians including Hippocrates understood the brain to be the center of thought, probably due to their experience observing the effects of head trauma.[182]
Medieval and Renaissance surgeons continued the practice of trepanation for head injury.[182] In the Middle Ages, physicians further described head injury symptoms and the term concussion became more widespread.[183] Concussion symptoms were first described systematically in the 16th century by Berengario da Carpi.[182]
It was first suggested in the 18th century that intracranial pressure rather than skull damage was the cause of pathology after TBI. This hypothesis was confirmed around the end of the 19th century, and opening the skull to relieve pressure was then proposed as a treatment.[179]
In the 19th century it was noted that TBI is related to the development of psychosis.[184] At that time a debate arose around whether post-concussion syndrome was due to a disturbance of the brain tissue or psychological factors.[183] The debate continues today.

Perhaps the first reported case of personality change after brain injury is that of Phineas Gage, who survived an accident in which a large iron rod was driven through his head, destroying one or both of his frontal lobes; numerous cases of personality change after brain injury have been reported since.[31][33][34][43][44][48][185][186]
The 20th century saw the advancement of technologies that improved treatment and diagnosis such as the development of imaging tools including CT and MRI, and, in the 21st century, diffusion tensor imaging (DTI). The introduction of intracranial pressure monitoring in the 1950s has been credited with beginning the "modern era" of head injury.[116][187] Until the 20th century, the mortality rate of TBI was high and rehabilitation was uncommon; improvements in care made during World War I reduced the death rate and made rehabilitation possible.[178] Facilities dedicated to TBI rehabilitation were probably first established during World War I.[178] Explosives used in World War I caused many blast injuries; the large number of TBIs that resulted allowed researchers to learn about localization of brain functions.[188] Blast-related injuries are now common problems in returning veterans from Iraq & Afghanistan; research shows that the symptoms of such TBIs are largely the same as those of TBIs involving a physical blow to the head.[189]
In the 1970s, awareness of TBI as a public health problem grew,[190] and a great deal of progress has been made since then in brain trauma research,[116] such as the discovery of primary and secondary brain injury.[179] The 1990s saw the development and dissemination of standardized guidelines for treatment of TBI, with protocols for a range of issues such as drugs and management of intracranial pressure.[116] Research since the early 1990s has improved TBI survival;[179] that decade was known as the "Decade of the Brain" for advances made in brain research.[191]
Research directions
Diagnosis
Quantitative EEG and EEG, which has no specific patterns in TBI is used in research settings to differentiate between mild TBI and no TBI.[192]
Medications
As of 2008, no medication is approved to halt the progression of the initial injury to secondary injury.[63] The variety of pathological events presents opportunities to find treatments that interfere with the damage processes.[10]
Further research is necessary to determine if the vasoconstrictor indomethacin (indometacin) can be used to treat increased pressure in the skull following a TBI.[193] In addition, drugs such as NMDA receptor antagonists to halt neurochemical cascades such as excitotoxicity showed promise in animal trials but failed in clinical trials.[116] These failures could be due to factors including faults in the trial designs or in the insufficiency of a single agent to prevent the array of injury processes involved in secondary injury.[116] Other topics of research have included investigations into mannitol,[103] dexamethasone,[194] barbiturates,[195] magnesium (no strong evidence),[196] and calcium channel blockers.[197]
Procedures
Although neuroprotection methods to decrease secondary injury have been the subject of interest follows TBI, trials to test agents that could halt these cellular mechanisms have met largely with failure as of 2008.[10] For example, interest existed in cooling the injured brain; however, a 2020 Cochrane review did not find enough evidence to see if it was useful or not.[198] Maintaining a normal temperature in the immediate period after a TBI appeared useful.[199] One review found a lower than normal temperature was useful in adults but not children.[200] While two other reviews found it did not appear to be useful.[201][199] In addition to traditional imaging modalities, there are several devices that help to monitor brain injury and facilitate research. Microdialysis allows ongoing sampling of extracellular fluid for analysis of metabolites that might indicate ischemia or brain metabolism, such as glucose, glycerol, and glutamate.[202][203] Intraparenchymal brain tissue oxygen monitoring systems (either Licox or Neurovent-PTO) are used routinely in neurointensive care in the US.[204] A non invasive model called CerOx is in development.[205]
Research is also planned to clarify factors correlated to outcome in TBI and to determine in which cases it is best to perform CT scans and surgical procedures.[206]
Hyperbaric oxygen therapy (HBO) has been evaluated as an add on treatment following TBI. The findings of a 2012 Cochrane systematic review does not justify the routine use of hyperbaric oxygen therapy to treat people recovering from a traumatic brain injury.[207] This review also reported that only a small number of randomized controlled trials had been conducted at the time of the review, many of which had methodological problems and poor reporting.[207] HBO for TBI is controversial with further evidence required to determine if it has a role.[208][207]
Psychological
Further research is required to determine the effectiveness of non-pharmacological treatment approaches for treating depression in children/adolescents and adults with TBI.[209]
As of 2010, the use of predictive visual tracking measurement to identify mild traumatic brain injury was being studied. In visual tracking tests, a head-mounted display unit with eye-tracking capability shows an object moving in a regular pattern. People without brain injury are able to track the moving object with smooth pursuit eye movements and correct trajectory. The test requires both attention and working memory which are difficult functions for people with mild traumatic brain injury. The question being studied, is whether results for people with brain injury will show visual-tracking gaze errors relative to the moving target.[210]
Monitoring pressure
Pressure reactivity index is used to correlate intracranial pressure with arterial blood pressure to give information about the state of cerebral perfusion, thus guiding treatment and prevent excessively high or low blood flow to the brain.[211] However, such method of monitoring intracranial pressure of equal or less than 20 mmHg is no better than imaging and clinical examination that monitor the neurological status of the brain in prolonging the survival, preserving the mental or functional status of the subject.[212]
Sensory processing
In animal models of TBI, sensory processing has been widely studied to show systematic defects arise and are slowly but likely only partially recovered.[213] It is especially characterised by an initial period of decreased activity in upper cortical layers.[214][215] This period of decreased activity has also been characterised as by specific timing effects in the patterns of cortical activity in these upper layers in response to regular sensory stimuli.[216]
References
- ^ Jones DK (2010). Diffusion MRI. Oxford University Press. p. 25. ISBN 978-0-19-970870-3.
- ^ a b Rehman T, Ali R, Tawil I, Yonas H (October 2008). "Rapid progression of traumatic bifrontal contusions to transtentorial herniation: A case report". Cases Journal. 1 (1): 203. doi:10.1186/1757-1626-1-203. PMC 2566562. PMID 18831756.
- ^ a b c "TBI: Get the Facts". CDC. March 11, 2019. Retrieved May 28, 2019.
- ^ "Traumatic Brain Injury". medlineplus.gov. Retrieved May 28, 2019.
- ^ a b Silverberg ND, Iverson GL, ACRM Brain Injury Special Interest Group Mild TBI Task Force and the ACRM Mild TBI Definition Expert Consensus Group, ACRM Brain Injury Special Interest Group Mild TBI Task Force members, Cogan A, Dams-O'Connor K, Delmonico R, Graf MJ, Iaccarino MA, Kajankova M, Kamins J, McCulloch KL, McKinney G, Nagele D, Panenka WJ, Rabinowitz AR (May 19, 2023). "The American Congress of Rehabilitation Medicine Diagnostic Criteria for Mild Traumatic Brain Injury" (PDF). Archives of Physical Medicine and Rehabilitation. 104 (8): S0003–9993(23)00297–6. doi:10.1016/j.apmr.2023.03.036. ISSN 1532-821X. PMID 37211140. S2CID 258821806.
- ^ "Basic Information about Traumatic Brain Injury | Concussion | Traumatic Brain Injury | CDC Injury Center". www.cdc.gov. March 6, 2019. Retrieved July 21, 2020.
- ^ "Prevention". CDC. March 4, 2019. Retrieved May 28, 2019.
- ^ a b Alves OL, Bullock R (2001). "Excitotoxic damage in traumatic brain injury". In Clark RS, Kochanek P (eds.). Brain injury. Boston: Kluwer Academic Publishers. p. 1. ISBN 978-0-7923-7532-6.
- ^ a b c d Rao V, Lyketsos C (2000). "Neuropsychiatric sequelae of traumatic brain injury". Psychosomatics. 41 (2): 95–103. doi:10.1176/appi.psy.41.2.95. PMID 10749946.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y Maas AI, Stocchetti N, Bullock R (August 2008). "Moderate and severe traumatic brain injury in adults". The Lancet. Neurology. 7 (8): 728–741. doi:10.1016/S1474-4422(08)70164-9. PMID 18635021. S2CID 14071224.
- ^ a b c d e f g h i j k l m n o Parikh S, Koch M, Narayan RK (2007). "Traumatic brain injury". International Anesthesiology Clinics. 45 (3): 119–135. doi:10.1097/AIA.0b013e318078cfe7. PMID 17622833. S2CID 46012183.
- ^ Chapman SB, Levin HS, Lawyer SL (1999). "Communication problems resulting from brain injury in children: Special issues of assessment and management". In McDonald S, Togher L, Code C (eds.). Communication Disorders Following Traumatic Brain Injury. East Sussex: Psychology Press. pp. 235–36. ISBN 978-0-86377-724-0.
- ^ a b Collins C, Dean J (2002). "Acquired brain injury". In Turner A, Foster M, Johnson SE (eds.). Occupational Therapy and Physical Dysfunction: Principles, Skills and Practice. Edinburgh: Churchill Livingstone. pp. 395–96. ISBN 978-0-443-06224-7.[permanent dead link]
- ^ a b Blissitt PA (September 2006). "Care of the critically ill patient with penetrating head injury". Critical Care Nursing Clinics of North America. 18 (3): 321–332. doi:10.1016/j.ccell.2006.05.006. PMID 16962454.
- ^ a b c d e f g h i Hannay HJ, Howieson DB, Loring DW, Fischer JS, Lezak MD (2004). "Neuropathology for neuropsychologists". In Lezak MD, Howieson DB, Loring DW (eds.). Neuropsychological Assessment. Oxford [Oxfordshire]: Oxford University Press. pp. 158–62. ISBN 978-0-19-511121-7.
- ^ a b c Jennett B (May 1998). "Epidemiology of head injury". Archives of Disease in Childhood. 78 (5): 403–406. doi:10.1136/adc.78.5.403. PMC 1717568. PMID 9659083.
- ^ McCaffrey RJ (1997). "Special issues in the evaluation of mild traumatic brain injury". The Practice of Forensic Neuropsychology: Meeting Challenges in the Courtroom. New York: Plenum Press. pp. 71–75. ISBN 978-0-306-45256-7.
- ^ a b LaPlaca et al. (2007). p. 16
- ^ Weber JT, Maas AI (2007). Weber JT (ed.). Neurotrauma: New Insights Into Pathology and Treatment. Amsterdam: Academic Press. p. xi. ISBN 978-0-444-53017-2.
- ^ a b c d Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT (July 2008). "Classification of traumatic brain injury for targeted therapies". Journal of Neurotrauma. 25 (7): 719–738. doi:10.1089/neu.2008.0586. PMC 2721779. PMID 18627252.
- ^ a b c Department of Defense and Department of Veterans Affairs (2008). "DoD/VA CODE PROPOSAL FINAL—508 COMPLIANT" (PDF).
- ^ Marion (1999). p. 4.
- ^ a b c d e f g h i j k l m n o p Valadka AB (2004). "Injury to the cranium". In Moore EJ, Feliciano DV, Mattox KL (eds.). Trauma. New York: McGraw-Hill, Medical Pub. Division. pp. 385–406. ISBN 978-0-07-137069-1.
- ^ Seidenwurm DI (2007). "Introduction to brain imaging". In Brant WE, Helms CA (eds.). Fundamentals of Diagnostic Radiology. Philadelphia: Lippincott, Williams & Wilkins. pp. 53–55. ISBN 978-0-7817-6135-2.
- ^ a b c Smith DH, Meaney DF, Shull WH (2003). "Diffuse axonal injury in head trauma". The Journal of Head Trauma Rehabilitation. 18 (4): 307–316. doi:10.1097/00001199-200307000-00003. PMID 16222127. S2CID 1527912.
- ^ Granacher (2007). p. 32.
- ^ Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM (October 2007). "White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study". Brain. 130 (Pt 10): 2508–2519. doi:10.1093/brain/awm216. PMID 17872928.
- ^ Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, et al. (April 2009). "Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function". Journal of Neurotrauma. 26 (4): 481–495. doi:10.1089/neu.2008.0461. PMID 19196176.
- ^ Melvin JW, Lighthall JW (2002). Nahum AM, Melvin JW (eds.). Accidental Injury: Biomechanics and Prevention. Berlin: Springer. pp. 280–81. ISBN 978-0-387-98820-7.
brain injury biomechanics
- ^ McCrea M (2007). Mild Traumatic Brain Injury and Postconcussion Syndrome: The New Evidence Base for Diagnosis and Treatment. American Academy of Clinical Neuropsychology Workshop Series. New York: Oxford University Press. ISBN 978-0-19-532829-5.
- ^ a b c Mattson AJ, Levin HS (May 1990). "Frontal lobe dysfunction following closed head injury. A review of the literature". The Journal of Nervous and Mental Disease. 178 (5): 282–291. doi:10.1097/00005053-199005000-00002. PMID 2187053. S2CID 27836314.
- ^ Bayly PV, Cohen TS, Leister EP, Ajo D, Leuthardt EC, Genin GM (August 2005). "Deformation of the human brain induced by mild acceleration". Journal of Neurotrauma. 22 (8): 845–856. doi:10.1089/neu.2005.22.845. PMC 2377024. PMID 16083352.
- ^ a b Cummings JL (August 1993). "Frontal-subcortical circuits and human behavior". Archives of Neurology. 50 (8): 873–880. doi:10.1001/archneur.1993.00540080076020. PMID 8352676.
- ^ a b c McDonald S, Flanagan S, Rollins J, Kinch J (2003). "TASIT: A new clinical tool for assessing social perception after traumatic brain injury". The Journal of Head Trauma Rehabilitation. 18 (3): 219–238. doi:10.1097/00001199-200305000-00001. PMID 12802165. S2CID 22361889.
- ^ Basso A, Scarpa MT (December 1990). "Traumatic aphasia in children and adults: a comparison of clinical features and evolution". Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 26 (4): 501–514. doi:10.1016/s0010-9452(13)80300-0. PMID 1706973. S2CID 4477808.
- ^ Mohr JP, Weiss GH, Caveness WF, Dillon JD, Kistler JP, Meirowsky AM, Rish BL (December 1980). "Language and motor disorders after penetrating head injury in Viet Nam". Neurology. 30 (12): 1273–1279. doi:10.1212/wnl.30.12.1273. PMID 7192808. S2CID 25106246.
- ^ a b c d e f g h Hardman JM, Manoukian A (May 2002). "Pathology of head trauma". Neuroimaging Clinics of North America. 12 (2): 175–87, vii. doi:10.1016/S1052-5149(02)00009-6. PMID 12391630.
TBI is highest in young adults aged 15 to 24 years and higher in men than women in all age groups.
- ^ a b Barkley JM, Morales D, Hayman LA, Diaz-Marchan PJ (2006). "Static neuroimaging in the evaluation of TBI". In Zasler ND, Katz DI, Zafonte RD (eds.). Brain Injury Medicine: Principles and Practice. Demos Medical Publishing. pp. 140–43. ISBN 978-1-888799-93-4.
- ^ a b c d e f g h Ghajar J (September 2000). "Traumatic brain injury". Lancet. 356 (9233): 923–929. doi:10.1016/S0140-6736(00)02689-1. PMID 11036909. S2CID 45288155.
- ^ Arlinghaus KA, Shoaib AM, Price TR (2005). "Neuropsychiatric assessment". In Silver JM, McAllister TW, Yudofsky SC (eds.). Textbook of Traumatic Brain Injury. Washington, DC: American Psychiatric Association. pp. 63–65. ISBN 978-1-58562-105-7.
- ^ a b c d e "NINDS Traumatic Brain Injury Information Page". National Institute of Neurological Disorders and Stroke. September 15, 2008. Archived from the original on December 3, 2016. Retrieved October 27, 2008.
- ^ a b Kushner D (1998). "Mild traumatic brain injury: toward understanding manifestations and treatment". Archives of Internal Medicine. 158 (15): 1617–1624. doi:10.1001/archinte.158.15.1617. PMID 9701095.
- ^ a b Stone VE, Baron-Cohen S, Knight RT (September 1998). "Frontal lobe contributions to theory of mind". Journal of Cognitive Neuroscience. 10 (5): 640–656. CiteSeerX 10.1.1.330.1488. doi:10.1162/089892998562942. PMID 9802997. S2CID 207724498.
- ^ a b Kim E (2002). "Agitation, aggression, and disinhibition syndromes after traumatic brain injury". NeuroRehabilitation. 17 (4): 297–310. doi:10.3233/NRE-2002-17404. PMID 12547978.
- ^ Busch RM, McBride A, Curtiss G, Vanderploeg RD (November 2005). "The components of executive functioning in traumatic brain injury". Journal of Clinical and Experimental Neuropsychology. 27 (8): 1022–1032. doi:10.1080/13803390490919263. PMID 16207623. S2CID 8840941.
- ^ a b Ponsford J, Draper K, Schönberger M (March 2008). "Functional outcome 10 years after traumatic brain injury: its relationship with demographic, injury severity, and cognitive and emotional status". Journal of the International Neuropsychological Society. 14 (2): 233–242. doi:10.1017/S1355617708080272. PMID 18282321.
- ^ Williams C, Wood RL (March 2010). "Alexithymia and emotional empathy following traumatic brain injury". Journal of Clinical and Experimental Neuropsychology. 32 (3): 259–267. doi:10.1080/13803390902976940. PMID 19548166. S2CID 34126700.
- ^ a b Milders M, Fuchs S, Crawford JR (April 2003). "Neuropsychological impairments and changes in emotional and social behaviour following severe traumatic brain injury". Journal of Clinical and Experimental Neuropsychology. 25 (2): 157–172. CiteSeerX 10.1.1.510.871. doi:10.1076/jcen.25.2.157.13642. PMID 12754675. S2CID 2264964.
- ^ Ownsworth T, Fleming J (2005). "The relative importance of metacognitive skills, emotional status, and executive function in psychosocial adjustment following acquired brain injury". The Journal of Head Trauma Rehabilitation. 20 (4): 315–332. doi:10.1097/00001199-200507000-00004. PMID 16030439. S2CID 41271134.
- ^ Dahlberg CA, Cusick CP, Hawley LA, Newman JK, Morey CE, Harrison-Felix CL, Whiteneck GG (December 2007). "Treatment efficacy of social communication skills training after traumatic brain injury: a randomized treatment and deferred treatment controlled trial". Archives of Physical Medicine and Rehabilitation. 88 (12): 1561–1573. doi:10.1016/j.apmr.2007.07.033. PMID 18047870.
- ^ a b c d e f g Salomone JP, Frame SB (2004). "Prehospital care". In Moore EJ, Feliciano DV, Mattox KL (eds.). Trauma. New York: McGraw-Hill, Medical Pub. Division. pp. 117–18. ISBN 978-0-07-137069-1.
- ^ a b "Signs and Symptoms". Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. July 7, 2007. Retrieved October 27, 2008.
- ^ Faul M, Xu L, Wald MM, Coronado VG (2010). "Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths, 2002–2006". National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Retrieved October 22, 2013.
- ^ a b Reilly P (2007). "The impact of neurotrauma on society: An international perspective". In Weber JT (ed.). Neurotrauma: New Insights Into Pathology and Treatment. Amsterdam: Academic Press. pp. 5–7. ISBN 978-0-444-53017-2.
- ^ "Get the Facts About TBI | Concussion | Traumatic Brain Injury | CDC Injury Center". www.cdc.gov. April 20, 2023. Retrieved April 1, 2024.
- ^ a b c d "Traumatic brain injury". Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2007. Retrieved October 28, 2008.
- ^ Granacher (2007). p. 16.
- ^ Hunt JP, Weintraub SL, Wang YZ, Buetcher KJ (2004). "Kinematics of trauma". In Moore EJ, Feliciano DV, Mattox KL (eds.). Trauma. New York: McGraw-Hill, Medical Pub. Division. p. 153. ISBN 978-0-07-137069-1.
- ^ a b Elovic E, Zafonte R (2005). "Prevention". In Silver JM, McAllister TW, Yudofsky SC (eds.). Textbook of Traumatic Brain Injury. Washington, DC: American Psychiatric Association. p. 740. ISBN 978-1-58562-105-7.
- ^ Bay E, McLean SA (February 2007). "Mild traumatic brain injury: an update for advanced practice nurses". The Journal of Neuroscience Nursing. 39 (1): 43–51. doi:10.1097/01376517-200702000-00009. PMID 17396538. S2CID 44600297.
- ^ a b Comper P, Bisschop SM, Carnide N, Tricco A (October 2005). "A systematic review of treatments for mild traumatic brain injury". Brain Injury. 19 (11): 863–880. doi:10.1080/02699050400025042. PMID 16296570. S2CID 34912966.
- ^ Champion HR, Holcomb JB, Young LA (May 2009). "Injuries from explosions: physics, biophysics, pathology, and required research focus". The Journal of Trauma. 66 (5): 1468–77, discussion 1477. doi:10.1097/TA.0b013e3181a27e7f. PMID 19430256. Archived from the original on September 23, 2017. Retrieved May 16, 2018.
- ^ a b c d e f g h i j Park E, Bell JD, Baker AJ (April 2008). "Traumatic brain injury: can the consequences be stopped?". CMAJ. 178 (9): 1163–1170. doi:10.1503/cmaj.080282. PMC 2292762. PMID 18427091.
- ^ "Pentagon Told Congress It's Studying Brain-Damage Therapy". ProPublica. December 23, 2010. Retrieved January 23, 2011.
Brave Americans who risked everything for their country and sustained traumatic brain injuries – the signature injury of the wars in Iraq and Afghanistan – deserve cognitive rehabilitation therapy to help them secure the best futures possible. It is unacceptable that the United States has been at war for nearly a decade and there is still no plan to treat these soldiers.
- ^ a b Thornton KE, Carmody DP (June 2008). "Efficacy of traumatic brain injury rehabilitation: interventions of QEEG-guided biofeedback, computers, strategies, and medications". Applied Psychophysiology and Biofeedback. 33 (2): 101–24. doi:10.1007/s10484-008-9056-z. PMID 18551365. S2CID 7262160.
- ^ Thornton KE, Carmody DP (March 2009). "Traumatic brain injury rehabilitation: QEEG biofeedback treatment protocols". Applied Psychophysiology and Biofeedback. 34 (1): 59–68. doi:10.1007/s10484-009-9075-4. PMID 19199027. S2CID 7928780.
- ^ a b Shaw NA (July 2002). "The neurophysiology of concussion". Progress in Neurobiology. 67 (4): 281–344. doi:10.1016/S0301-0082(02)00018-7. PMID 12207973. S2CID 46514293.
- ^ American Academy of Pediatrics: Committee on Child Abuse and Neglect (July 2001). "Shaken baby syndrome: rotational cranial injuries-technical report". Pediatrics. 108 (1): 206–210. doi:10.1542/peds.108.1.206. PMID 11433079.
- ^ Morrison AL, King TM, Korell MA, Smialek JE, Troncoso JC (June 1998). "Acceleration-deceleration injuries to the brain in blunt force trauma". The American Journal of Forensic Medicine and Pathology. 19 (2): 109–112. doi:10.1097/00000433-199806000-00002. PMID 9662103.
- ^ Poirier MP (2003). "Concussions: Assessment, management, and recommendations for return to activity". Clinical Pediatric Emergency Medicine. 4 (3): 179–85. doi:10.1016/S1522-8401(03)00061-2.
- ^ Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT (February 1995). "Epidemiology of trauma deaths: a reassessment". The Journal of Trauma. 38 (2): 185–193. doi:10.1097/00005373-199502000-00006. PMID 7869433.
- ^ a b Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, et al. (May 2002). "Clinical trials in head injury". Journal of Neurotrauma. 19 (5): 503–557. doi:10.1089/089771502753754037. PMC 1462953. PMID 12042091.
- ^ Xiong Y, Lee CP, Peterson PL (2000). "Mitochondrial dysfunction following traumatic brain injury". In Miller LP, Hayes RL, Newcomb JK (eds.). Head Trauma: Basic, Preclinical, and Clinical Directions. New York: John Wiley and Sons, Inc. pp. 257–80. ISBN 978-0-471-36015-5.
- ^ Scalea TM (2005). "Does it matter how head injured patients are resuscitated?". In Valadka AB, Andrews BT (eds.). Neurotrauma: Evidence-based Answers to Common Questions. Thieme. pp. 3–4. ISBN 978-3-13-130781-1.
- ^ Morley EJ, Zehtabchi S (September 2008). "Evidence-based emergency medicine/systematic review abstract. Mannitol for traumatic brain injury: searching for the evidence". Annals of Emergency Medicine. 52 (3): 298–300. doi:10.1016/j.annemergmed.2007.10.013. PMID 18763356.
- ^ a b c d e f g h i j Zink BJ (March 2001). "Traumatic brain injury outcome: concepts for emergency care". Annals of Emergency Medicine. 37 (3): 318–332. doi:10.1067/mem.2001.113505. PMID 11223769.
- ^ Wortzel HS, Arciniegas DB (January 1, 2014). "The DSM-5 approach to the evaluation of traumatic brain injury and its neuropsychiatric sequelae". NeuroRehabilitation. 34 (4): 613–623. doi:10.3233/NRE-141086. PMID 24820171.
- ^ Simpson JR (June 1, 2014). "DSM-5 and neurocognitive disorders". The Journal of the American Academy of Psychiatry and the Law. 42 (2): 159–164. PMID 24986342.
- ^ "The DSM-5 Approach to Evaluating Traumatic Brain Injury". www.hsrd.research.va.gov. Retrieved November 5, 2019.
- ^ a b Barr RM, Gean AD, Le TH (2007). "Craniofacial trauma". In Brant WE, Helms CA (eds.). Fundamentals of Diagnostic Radiology. Philadelphia: Lippincott, Williams & Wilkins. p. 55. ISBN 978-0-7817-6135-2.
- ^ "University Times ' Research Notes". University of Pittsburgh. March 22, 2012. Retrieved November 10, 2013.
- ^ Coles JP (July 2007). "Imaging after brain injury". British Journal of Anaesthesia. 99 (1): 49–60. doi:10.1093/bja/aem141. PMID 17573394.
- ^ "Diagnosis and Assessment of Traumatic Brain Injury". Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans. National Academies Press (US). April 10, 2019.
- ^ "Traumatic Brain Injury: What to Know About Symptoms, Diagnosis, and Treatment". Fda.gov. August 23, 2021.
- ^ From Flight magazine (1912), as described by Grothe S (March 26, 2016). "Mit dem Kopf durch die Wand (With the Head Through the Wall)". Der Spiegel (in German). Archived from the original on May 23, 2018.
- ^ Liu BC, Ivers R, Norton R, Boufous S, Blows S, Lo SK (January 2008). Liu BC (ed.). "Helmets for preventing injury in motorcycle riders". The Cochrane Database of Systematic Reviews. 4 (1): CD004333. doi:10.1002/14651858.CD004333.pub3. hdl:10536/DRO/DU:30009360. PMID 18254047.
- ^ McCrory PR (August 2003). "Brain injury and heading in soccer". BMJ. 327 (7411): 351–352. doi:10.1136/bmj.327.7411.351. PMC 1126775. PMID 12919964.
- ^ a b McIntosh AS, McCrory P (June 2005). "Preventing head and neck injury". British Journal of Sports Medicine. 39 (6): 314–318. doi:10.1136/bjsm.2005.018200. PMC 1725244. PMID 15911597.
- ^ a b c Crooks CY, Zumsteg JM, Bell KR (November 2007). "Traumatic brain injury: a review of practice management and recent advances". Physical Medicine and Rehabilitation Clinics of North America. 18 (4): 681–710, vi. doi:10.1016/j.pmr.2007.06.005. PMID 17967360.
- ^ Hahn RA, Bilukha O, Crosby A, Fullilove MT, Liberman A, Moscicki E, et al. (February 2005). "Firearms laws and the reduction of violence: a systematic review". American Journal of Preventive Medicine. 28 (2 Suppl 1): 40–71. doi:10.1016/j.amepre.2004.10.005. PMID 15698747.
- ^ Kluger, Jeffrey. "Dealing with Brain Injuries. Time Magazine, April 6, 2009, p. 57. Online: [1]. Accessed: May 1, 2009
- ^ a b c d e f g h i j k l Office of Communications and Public Liaison (February 2002). "Traumatic brain injury: Hope through research". NIH Publication No. 02-2478. National Institute of Neurological Disorders and Stroke, National Institutes of Health. Archived from the original on December 18, 2016. Retrieved August 17, 2008.
Many patients with mild to moderate head injuries who experience cognitive deficits become easily confused or distracted and have problems with concentration and attention. They also have problems with higher level, so-called executive functions, such as planning, organizing, abstract reasoning, problem solving, and making judgments, which may make it difficult to resume pre-injury work-related activities. Recovery from cognitive deficits is greatest within the first 6 months after the injury and more gradual after that.
- ^ Kawada T (2020). "Efficacy of tranexamic acid in patients with traumatic brain injury". EXCLI Journal. 19: 1547–1548. doi:10.17179/excli2020-3161. PMC 7744966. PMID 33343272.
- ^ a b Greenwald BD, Burnett DM, Miller MA (March 2003). "Congenital and acquired brain injury. 1. Brain injury: epidemiology and pathophysiology". Archives of Physical Medicine and Rehabilitation. 84 (3 Suppl 1): S3–S7. doi:10.1053/apmr.2003.50052. PMID 12708551.
- ^ a b Moppett IK (July 2007). "Traumatic brain injury: assessment, resuscitation and early management". British Journal of Anaesthesia. 99 (1): 18–31. doi:10.1093/bja/aem128. PMID 17545555. Moppet07
- ^ Wang F, Wang Y, Sun T, Yu HL (May 2016). "Hyperbaric oxygen therapy for the treatment of traumatic brain injury: a meta-analysis". Neurological Sciences. 37 (5): 693–701. doi:10.1007/s10072-015-2460-2. PMID 26746238. S2CID 10548255.
- ^ Alarcon JD, Rubiano AM, Okonkwo DO, Alarcón J, Martinez-Zapata MJ, Urrútia G, Bonfill Cosp X (December 2017). "Elevation of the head during intensive care management in people with severe traumatic brain injury". The Cochrane Database of Systematic Reviews. 2017 (12): CD009986. doi:10.1002/14651858.CD009986.pub2. PMC 6486002. PMID 29283434.
- ^ Gruen P (May 2002). "Surgical management of head trauma". Neuroimaging Clinics of North America. 12 (2): 339–343. doi:10.1016/S1052-5149(02)00013-8. PMID 12391640.
- ^ a b c d Gultekin R, Huang S, Clavisi O, Pattuwage L, König TC, Gruen R (March 2016). "Pharmacological interventions in traumatic brain injury: Can we rely on systematic reviews for evidence?". Injury. 47 (3): 516–524. doi:10.1016/j.injury.2015.10.011. PMID 26589595.
- ^ a b Burgess S, Abu-Laban RB, Slavik RS, Vu EN, Zed PJ (April 2016). "A Systematic Review of Randomized Controlled Trials Comparing Hypertonic Sodium Solutions and Mannitol for Traumatic Brain Injury: Implications for Emergency Department Management". The Annals of Pharmacotherapy. 50 (4): 291–300. doi:10.1177/1060028016628893. PMID 26825644. S2CID 23859003.
- ^ Mathur A, Duke T, Kukuruzovic R, South M (2004). "Hypotonic vs isotonic saline solutions for intravenous fluid management of acute infections". The Cochrane Database of Systematic Reviews. 2003 (2): CD004169. doi:10.1002/14651858.CD004169.pub2. PMC 6986696. PMID 15106241.
- ^ Rickard AC, Smith JE, Newell P, Bailey A, Kehoe A, Mann C (August 2014). "Salt or sugar for your injured brain? A meta-analysis of randomised controlled trials of mannitol versus hypertonic sodium solutions to manage raised intracranial pressure in traumatic brain injury". Emergency Medicine Journal. 31 (8): 679–683. doi:10.1136/emermed-2013-202679. hdl:10026.1/4537. PMID 23811861. S2CID 24947684.
- ^ a b Wakai A, McCabe A, Roberts I, Schierhout G (August 2013). "Mannitol for acute traumatic brain injury". The Cochrane Database of Systematic Reviews. 8 (8): CD001049. doi:10.1002/14651858.CD001049.pub5. PMC 7050611. PMID 23918314.
- ^ Chen H, Song Z, Dennis JA (January 2020). "Hypertonic saline versus other intracranial pressure-lowering agents for people with acute traumatic brain injury". The Cochrane Database of Systematic Reviews. 1 (1): CD010904. doi:10.1002/14651858.CD010904.pub3. PMC 6984412. PMID 31978260.
- ^ Roberts I, Smith R, Evans S (February 2007). "Doubts over head injury studies". BMJ. 334 (7590): 392–394. doi:10.1136/bmj.39118.480023.BE. PMC 1804156. PMID 17322250.
- ^ Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, Carson S, Chesnut RM, Ghajar J, Goldstein B, Grant GA, Kissoon N, Peterson K, Selden NR, Tasker RC, Tong KA, Vavilala MS, Wainwright MS, Warden CR, American Academy of Pediatrics-Section on Neurological Surgery, American Association of Neurological Surgeons/Congress of Neurological Surgeons, Child Neurology Society, European Society of Pediatric and Neonatal Intensive Care, Neurocritical Care Society, Pediatric Neurocritical Care Research Group, Society of Critical Care Medicine, Paediatric Intensive Care Society UK, Society for Neuroscience in Anesthesiology and Critical Care, World Federation of Pediatric Intensive and Critical Care Societies (January 2012). "Guidelines for the Acute Medical Management of Severe Traumatic Brain Injury in Infants, Children, and Adolescents-Second Edition". Pediatric Critical Care Medicine. 13: S1–S2. doi:10.1097/PCC.0b013e31823f435c. ISSN 1529-7535. PMID 22217782.
- ^ Schierhout G, Roberts I (2000). "Hyperventilation therapy for acute traumatic brain injury". The Cochrane Database of Systematic Reviews. 1997 (2): CD000566. doi:10.1002/14651858.CD000566. PMC 7061354. PMID 10796728.
- ^ Curry R, Hollingworth W, Ellenbogen RG, Vavilala MS (March 2008). "Incidence of hypo- and hypercarbia in severe traumatic brain injury before and after 2003 pediatric guidelines". Pediatric Critical Care Medicine. 9 (2): 141–146. doi:10.1097/PCC.0B013e318166870e. PMID 18477926. S2CID 9927553.
- ^ Alderson P, Roberts I (January 2005). "Corticosteroids for acute traumatic brain injury". The Cochrane Database of Systematic Reviews. 2005 (1): CD000196. doi:10.1002/14651858.CD000196.pub2. PMC 7043302. PMID 15674869.
- ^ Campbell J (2018). International Trauma Life Support for Emergency Care Providers (8th Global ed.). Pearson. p. 221. ISBN 978-1292-17084-8.
- ^ Roberts I (2000). "Aminosteroids for acute traumatic brain injury". The Cochrane Database of Systematic Reviews. 2010 (4): CD001527. doi:10.1002/14651858.CD001527. PMC 8406780. PMID 11034722.
- ^ Ker K, Blackhall K (January 2008). "Beta-2 receptor antagonists for acute traumatic brain injury". The Cochrane Database of Systematic Reviews. 2008 (1): CD006686. doi:10.1002/14651858.CD006686.pub2. PMC 7387850. PMID 18254109.
- ^ Willis C, Lybrand S, Bellamy N (2004). "Excitatory amino acid inhibitors for traumatic brain injury". The Cochrane Database of Systematic Reviews. 2003 (1): CD003986. doi:10.1002/14651858.CD003986.pub2. PMC 6984678. PMID 14974051.
- ^ Forsyth RJ, Jayamoni B, Paine TC (October 2006). "Monoaminergic agonists for acute traumatic brain injury". The Cochrane Database of Systematic Reviews (4): CD003984. doi:10.1002/14651858.CD003984.pub2. PMID 17054192.
- ^ Tasker RC (2008). "Head and spinal cord trauma". In Nichols DG (ed.). Roger's Textbook of Pediatric Intensive Care (4th ed.). PA: Lippincott Williams & Wilkins. pp. 887–911. ISBN 978-0-7817-8275-3.
- ^ a b c d e f g Marshall LF (September 2000). "Head injury: recent past, present, and future". Neurosurgery. 47 (3): 546–561. doi:10.1097/00006123-200009000-00002. PMID 10981741.
- ^ Adams JP (2010). "Non-neurological complications of brain injury". In Adams JP, Bell D, McKinlay J (eds.). Neurocritical care : a guide to practical management. London: Springer. pp. 77–88. ISBN 978-1-84882-069-2.
- ^ O'Leary R, McKinlay J (2011). "Neurogenic pulmonary oedema". Continuing Education in Anaesthesia, Critical Care & Pain. 11 (3): 87–92. doi:10.1093/bjaceaccp/mkr006.
- ^ Wilkins, Lippincott Williams (2005). Strategies for Managing Multisystem Disorders. Hagerstwon, MD: Lippincott Williams & Wilkins. p. 370. ISBN 978-1-58255-423-5.
- ^ a b Dunn IF, Ellegala DB (2008). "Decompressive hemicraniectomy in the management of severe traumatic brain injury". In Bhardwaj A, Ellegala DB, Kirsch JR (eds.). Acute Brain and Spinal Cord Injury: Evolving Paradigms and Management. Informa Health Care. pp. 1–2. ISBN 978-1-4200-4794-3.
- ^ Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D'Urso P, Kossmann T, Ponsford J, Seppelt I, Reilly P, Wolfe R (April 2011). "Decompressive craniectomy in diffuse traumatic brain injury" (PDF). The New England Journal of Medicine. 364 (16): 1493–502. doi:10.1056/NEJMoa1102077. PMID 21434843.
- ^ Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J (January 2017). "Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition". Neurosurgery. 80 (1): 6–15. doi:10.1227/NEU.0000000000001432. PMID 27654000. S2CID 3616546.
- ^ Turner-Stokes L, Pick A, Nair A, Disler PB, Wade DT (December 2015). "Multi-disciplinary rehabilitation for acquired brain injury in adults of working age". The Cochrane Database of Systematic Reviews. 2015 (12): CD004170. doi:10.1002/14651858.CD004170.pub3. PMC 8629646. PMID 26694853.
- ^ a b Ontario Neurotrauma Foundation and Institut national d'excellence en santé et en services sociaux (2015). "Clinical Practice Guideline for the Rehabilitation of Adults with Modarate to Severe Traumatic Brain Injury". braininjuryguidelines.org. Retrieved April 27, 2020.
- ^ Soo C, Tate R (July 2007). "Psychological treatment for anxiety in people with traumatic brain injury". The Cochrane Database of Systematic Reviews (3): CD005239. doi:10.1002/14651858.CD005239.pub2. PMID 17636792.
- ^ a b c McMillan TM, Oddy M (2000). "Service provision for social disability and handicap after acquired brain injury". In Wood RL, McMillan TM (eds.). Neurobehavioural Disability and Social Handicap Following Traumatic Brain Injury. East Sussex: Psychology Press. pp. 267–68. ISBN 978-0-86377-889-6.
- ^ Deb S, Crownshaw T (January 2004). "The role of pharmacotherapy in the management of behaviour disorders in traumatic brain injury patients". Brain Injury. 18 (1): 1–31. doi:10.1080/0269905031000110463. PMID 14660233. S2CID 24775120.
- ^ a b Agrawal A, Timothy J, Pandit L, Manju M (July 2006). "Post-traumatic epilepsy: an overview". Clinical Neurology and Neurosurgery. 108 (5): 433–439. doi:10.1016/j.clineuro.2005.09.001. PMID 16225987. S2CID 2650670.
- ^ Rodda J, Graham HK (November 2001). "Classification of gait patterns in spastic hemiplegia and spastic diplegia: a basis for a management algorithm". European Journal of Neurology. 8 (Suppl 5): 98–108. doi:10.1046/j.1468-1331.2001.00042.x. PMID 11851738. S2CID 45860264.
- ^ a b Grunt S. "Geh-Orthesen bei Kindern mit Cerebralparese". Pediatrica. 18: 30–34.
- ^ Esquenazi A (2008). Hsu JD, Michael JW, Fisk JR (eds.). Assessment and orthotic management of gait dysfunction in individuals with brain injury (4 ed.). Philadelphia: Mosby Elsevier. pp. 441–447. ISBN 978-0-323-03931-4.
{{cite book}}:|periodical=ignored (help) - ^ Horst R (2005). Motorisches Strategietraining und PNF. Renata Horst. ISBN 978-3-13-151351-9.
- ^ Novacheck TF (2008). Hsu JD, Michael JW, Fisk JR (eds.). Orthoses for cerebral palsy. AAOS Atlas of Orthoses and Assistive Devices. Philadelphia. pp. 487–500. ISBN 978-0-323-03931-4.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Muñoz S (2018). "The new generation of AFOs". The O&P EDGE. 11. Archived from the original on June 6, 2021. Retrieved October 25, 2021.
- ^ Kerkum YL, Harlaar J, Buizer AI, van den Noort JC, Becher JG, Brehm MA (May 2016). "An individual approach for optimizing ankle-foot orthoses to improve mobility in children with spastic cerebral palsy walking with excessive knee flexion". Gait & Posture. 46: 104–111. doi:10.1016/j.gaitpost.2016.03.001. PMID 27131186.
- ^ Kerkum YL (2016). "The effect of ankle foot orthosis stiffness on trunk movement and walking energy cost in cerebral palsy". Gait & Posture. 49: 2. doi:10.1016/j.gaitpost.2016.07.070. ISSN 0966-6362.
- ^ Meyns P, Kerkum YL, Brehm MA, Becher JG, Buizer AI, Harlaar J (May 2020). "Ankle foot orthoses in cerebral palsy: Effects of ankle stiffness on trunk kinematics, gait stability and energy cost of walking". European Journal of Paediatric Neurology. 26: 68–74. doi:10.1016/j.ejpn.2020.02.009. PMID 32147412. S2CID 212641072.
- ^ Waterval NF, Nollet F, Harlaar J, Brehm MA (October 2019). "Modifying ankle foot orthosis stiffness in patients with calf muscle weakness: gait responses on group and individual level". Journal of Neuroengineering and Rehabilitation. 16 (1): 120. doi:10.1186/s12984-019-0600-2. PMC 6798503. PMID 31623670.
- ^ Ploeger HE, Waterval NF, Nollet F, Bus SA, Brehm MA (2019). "Stiffness modification of two ankle-foot orthosis types to optimize gait in individuals with non-spastic calf muscle weakness - a proof-of-concept study". Journal of Foot and Ankle Research (in German). 12: 41. doi:10.1186/s13047-019-0348-8. PMC 6686412. PMID 31406508.
- ^ a b Brown AW, Elovic EP, Kothari S, Flanagan SR, Kwasnica C (March 2008). "Congenital and acquired brain injury. 1. Epidemiology, pathophysiology, prognostication, innovative treatments, and prevention". Archives of Physical Medicine and Rehabilitation. 89 (3 Suppl 1): S3–S8. doi:10.1016/j.apmr.2007.12.001. PMID 18295647.
- ^ a b Frey LC (2003). "Epidemiology of posttraumatic epilepsy: a critical review". Epilepsia. 44 (s10): 11–17. doi:10.1046/j.1528-1157.44.s10.4.x. PMID 14511389. S2CID 34749005.
- ^ Armin SS, Colohan AR, Zhang JH (June 2006). "Traumatic subarachnoid hemorrhage: our current understanding and its evolution over the past half century". Neurological Research. 28 (4): 445–452. doi:10.1179/016164106X115053. PMID 16759448. S2CID 23726077.
- ^ Chumney D, Nollinger K, Shesko K, Skop K, Spencer M, Newton RA (2010). "Ability of Functional Independence Measure to accurately predict functional outcome of stroke-specific population: systematic review". Journal of Rehabilitation Research and Development. 47 (1): 17–29. doi:10.1682/JRRD.2009.08.0140. PMID 20437324.
- ^ Murray ED, Buttner N, Price BH (2012). "Depression and Psychosis in Neurological Practice". In Bradley WG, Daroff RB, Fenichel GM, Jankovic J (eds.). Bradley's neurology in clinical practice. Vol. 1 (6th ed.). Philadelphia, PA: Elsevier/Saunders. p. 111. ISBN 978-1-4377-0434-1.
- ^ Juengst SB, Adams LM, Bogner JA, Arenth PM, O'Neil-Pirozzi TM, Dreer LE, et al. (November 2015). "Trajectories of life satisfaction after traumatic brain injury: Influence of life roles, age, cognitive disability, and depressive symptoms". Rehabilitation Psychology. 60 (4): 353–364. doi:10.1037/rep0000056. PMC 4667543. PMID 26618215.
- ^ Hassett L, Moseley AM, Harmer AR (December 2017). "Fitness training for cardiorespiratory conditioning after traumatic brain injury". The Cochrane Database of Systematic Reviews. 2017 (12): CD006123. doi:10.1002/14651858.CD006123.pub3. PMC 6486048. PMID 29286534.
- ^ "Persistent vegetative state" at Dorland's Medical Dictionary.
- ^ Schiff ND, Plum F, Rezai AR (March 2002). "Developing prosthetics to treat cognitive disabilities resulting from acquired brain injuries". Neurological Research. 24 (2): 116–124. doi:10.1179/016164102101199576. PMID 11877893. S2CID 347998.
- ^ a b Kwasnica C, Brown AW, Elovic EP, Kothari S, Flanagan SR (March 2008). "Congenital and acquired brain injury. 3. Spectrum of the acquired brain injury population". Archives of Physical Medicine and Rehabilitation. 89 (3 Suppl 1): S15–S20. doi:10.1016/j.apmr.2007.12.006. PMID 18295644.
- ^ Synnot A, Chau M, Pitt V, O'Connor D, Gruen RL, Wasiak J, et al. (November 2017). "Interventions for managing skeletal muscle spasticity following traumatic brain injury". The Cochrane Database of Systematic Reviews. 2017 (11): CD008929. doi:10.1002/14651858.CD008929.pub2. PMC 6486165. PMID 29165784.
- ^ Oliveros-Juste A, Bertol V, Oliveros-Cid A (2002). "[Preventive prophylactic treatment in posttraumatic epilepsy]". Revista de Neurología (in Spanish). 34 (5): 448–459. doi:10.33588/rn.3405.2001439. PMID 12040514.
- ^ Aimaretti G, Ghigo E (April 2007). "Should every patient with traumatic brain injury be referred to an endocrinologist?". Nature Clinical Practice. Endocrinology & Metabolism. 3 (4): 318–319. doi:10.1038/ncpendmet0460. PMID 17377615. S2CID 26338743.
- ^ Arlinghaus KA, Shoaib AM, Price TR (2005). "Neuropsychiatric assessment". In Silver JM, McAllister TW, Yudofsky SC (eds.). Textbook of Traumatic Brain Injury. Washington, DC: American Psychiatric Association. pp. 59–62. ISBN 978-1-58562-105-7.
- ^ Hall RC, Hall RC, Chapman MJ (2005). "Definition, diagnosis, and forensic implications of postconcussional syndrome". Psychosomatics. 46 (3): 195–202. doi:10.1176/appi.psy.46.3.195. PMID 15883140.
- ^ Jordan BD (2000). "Chronic traumatic brain injury associated with boxing". Seminars in Neurology. 20 (2): 179–185. doi:10.1055/s-2000-9826. PMID 10946737. S2CID 41087553.
- ^ Mendez MF (1995). "The neuropsychiatric aspects of boxing". International Journal of Psychiatry in Medicine. 25 (3): 249–262. doi:10.2190/CUMK-THT1-X98M-WB4C. PMID 8567192. S2CID 20238578.
- ^ a b c Draper K, Ponsford J (October 2009). "Long-term outcome following traumatic brain injury: a comparison of subjective reports by those injured and their relatives". Neuropsychological Rehabilitation. 19 (5): 645–661. doi:10.1080/17405620802613935. PMID 19629849. S2CID 205907369.
- ^ Rutherford GW, Corrigan JD (2009). "Long-term consequences of traumatic brain injury". The Journal of Head Trauma Rehabilitation. 24 (6): 421–423. doi:10.1097/HTR.0b013e3181c13439. PMID 19940674.
- ^ a b Temkin NR, Corrigan JD, Dikmen SS, Machamer J (2009). "Social functioning after traumatic brain injury". The Journal of Head Trauma Rehabilitation. 24 (6): 460–467. doi:10.1097/HTR.0b013e3181c13413. PMID 19940679. S2CID 43384334.
- ^ a b c Velikonja D, Warriner E, Brum C (July 2010). "Profiles of emotional and behavioral sequelae following acquired brain injury: cluster analysis of the Personality Assessment Inventory". Journal of Clinical and Experimental Neuropsychology. 32 (6): 610–621. doi:10.1080/13803390903401302. PMID 20029697. S2CID 21067999.
- ^ Hawley LA, Newman JK (2010). "Group interactive structured treatment (GIST): a social competence intervention for individuals with brain injury". Brain Injury. 24 (11): 1292–1297. doi:10.3109/02699052.2010.506866. PMID 20735320. S2CID 207447494.
- ^ Lane-Brown A, Tate R (April 2009). "Interventions for apathy after traumatic brain injury". The Cochrane Database of Systematic Reviews. 2009 (2): CD006341. doi:10.1002/14651858.CD006341.pub2. PMC 7390354. PMID 19370632.
- ^ Hesdorffer DC, Rauch SL, Tamminga CA (2009). "Long-term psychiatric outcomes following traumatic brain injury: a review of the literature". The Journal of Head Trauma Rehabilitation. 24 (6): 452–459. doi:10.1097/htr.0b013e3181c133fd. PMID 19940678. S2CID 205643570.
- ^ Fleminger S (2010). "Neuropsychiatric effects of traumatic brain injury". Psychiatr Times. 27 (3): 40–45. Archived from the original on March 16, 2020. Retrieved April 23, 2010.
- ^ Dockree PM, O'Keeffe FM, Moloney P, Bishara AJ, Carton S, Jacoby LL, Robertson IH (January 2006). "Capture by misleading information and its false acceptance in patients with traumatic brain injury". Brain. 129 (Pt 1): 128–140. doi:10.1093/brain/awh664. PMID 16280354.
- ^ Kreutzer JS, Stejskal TM, Ketchum JM, Marwitz JH, Taylor LA, Menzel JC (June 2009). "A preliminary investigation of the brain injury family intervention: impact on family members". Brain Injury. 23 (6): 535–547. doi:10.1080/02699050902926291. PMID 19484627. S2CID 12428952.
- ^ Kreutzer JS, Kolakowsky-Hayner SA, Demm SR, Meade MA (August 2002). "A structured approach to family intervention after brain injury". The Journal of Head Trauma Rehabilitation. 17 (4): 349–367. doi:10.1097/00001199-200208000-00008. PMID 12106003. S2CID 20918911.
- ^ a b c d León-Carrión J, Domínguez-Morales M, Barroso y Martín JM, Murillo-Cabezas F (2005). "Epidemiology of traumatic brain injury and subarachnoid hemorrhage". Pituitary. 8 (3–4): 197–202. doi:10.1007/s11102-006-6041-5. PMID 16508717. S2CID 23713764.
- ^ "Coma" at Dorland's Medical Dictionary.
- ^ Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L, et al. (WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury) (February 2004). "Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury". Journal of Rehabilitation Medicine. 36 (43 Suppl): 28–60. doi:10.1080/16501960410023732. PMID 15083870.
- ^ a b c D'Ambrosio R, Perucca E (December 2004). "Epilepsy after head injury". Current Opinion in Neurology. 17 (6): 731–735. doi:10.1097/00019052-200412000-00014. PMC 2672045. PMID 15542983.
- ^ Chesnutt RM, Eisenberg JM (1999). "Introduction and background". Rehabilitation for Traumatic Brain Injury. DIANE. p. 9. ISBN 978-0-7881-8376-8.
- ^ Tolias C, Sgouros S (February 4, 2005). "Initial evaluation and management of CNS injury". Medscape. Retrieved December 16, 2007.
- ^ Kindermann D, Mutter R, Pines JM (May 2013). Emergency Department Transfers to Acute Care Facilities 2009 (Report). Agency for Healthcare Research and Quality. HCUP Statistical Brief. Archived from the original on December 1, 2016.
- ^ Carli P, Orliaguet G (February 2004). "Severe traumatic brain injury in children". Lancet. 363 (9409): 584–585. doi:10.1016/S0140-6736(04)15626-2. PMID 14987880. S2CID 28381295.
- ^ Necajauskaite O, Endziniene M, Jureniene K (2005). "The prevalence, course and clinical features of post-concussion syndrome in children" (PDF). Medicina. 41 (6): 457–464. PMID 15998982. Archived from the original (PDF) on October 19, 2013. Retrieved January 26, 2007.
- ^ "An Unrecognized Problem" (PDF). Centers for Disease Control and Prevention. Retrieved October 13, 2019.
- ^ a b c Boake and Diller (2005). p. 3
- ^ a b c d Granacher (2007). p. 1.
- ^ Scurlock JA, Andersen BR (2005). Diagnoses in Assyrian and Babylonian Medicine: Ancient Sources, Translations, and Modern Medical Analyses. Urbana: University of Illinois Press. p. 307. ISBN 978-0-252-02956-1.
- ^ Sanchez GM, Burridge AL (2007). "Decision making in head injury management in the Edwin Smith Papyrus". Neurosurgical Focus. 23 (1): E5. doi:10.3171/FOC-07/07/E5. PMID 17961064.
- ^ a b c Levin HS, Benton AL, Grossman R (1982). "Historical review of head injury". Neurobehavioral Consequences of Closed Head Injury. Oxford [Oxfordshire]: Oxford University Press. pp. 3–5. ISBN 978-0-19-503008-2.
- ^ a b Zillmer EA, Schneider J, Tinker J, Kaminaris CI (2006). "A history of sports-related concussions: A neuropsychological perspective". In Echemendia RJ (ed.). Sports Neuropsychology: Assessment and Management of Traumatic Brain Injury. New York: The Guilford Press. pp. 21–23. ISBN 978-1-57230-078-1.
- ^ Corcoran C, McAlister TW, Malaspina D (2005). "Psychotic disorders". In Silver JM, McAllister TW, Yudofsky SC (eds.). Textbook of Traumatic Brain Injury. Washington, DC: American Psychiatric Association. p. 213. ISBN 978-1-58562-105-7.
- ^ Eslinger PJ, Damasio AR (December 1985). "Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR". Neurology. 35 (12): 1731–1741. doi:10.1212/wnl.35.12.1731. PMID 4069365. S2CID 22373825.
- ^ Devinsky O, D'Esposito M (2004). Neurology of Cognitive and Behavioral Disorders. Vol. 68. New York: Oxford University Press. ISBN 978-0-19-513764-4.
- ^ Marion (1999). p. 3.
- ^ Jones E, Fear NT, Wessely S (November 2007). "Shell shock and mild traumatic brain injury: a historical review". The American Journal of Psychiatry. 164 (11): 1641–1645. doi:10.1176/appi.ajp.2007.07071180. PMID 17974926.
- ^ Belanger HG, Kretzmer T, Yoash-Gantz R, Pickett T, Tupler LA (January 2009). "Cognitive sequelae of blast-related versus other mechanisms of brain trauma". Journal of the International Neuropsychological Society. 15 (1): 1–8. doi:10.1017/S1355617708090036. PMID 19128523. S2CID 21648436.
- ^ Boake and Diller (2005). p. 8
- ^ Bush GH (July 1990). "Project on the Decade of the Brain". Library of Congress. Retrieved October 22, 2013.
- ^ Livint Popa L, Dragos H, Pantelemon C, Verisezan Rosu O, Strilciuc S (2020). "The Role of Quantitative EEG in the Diagnosis of Neuropsychiatric Disorders". J Med Life. 13 (1): 8–15. doi:10.25122/jml-2019-0085. PMC 7175442. PMID 32341694.
- ^ Martín-Saborido C, López-Alcalde J, Ciapponi A, Sánchez Martín CE, Garcia Garcia E, Escobar Aguilar G, et al. (November 2019). "Indomethacin for intracranial hypertension secondary to severe traumatic brain injury in adults". The Cochrane Database of Systematic Reviews. 2019 (11). doi:10.1002/14651858.CD011725.pub2. PMC 6872435. PMID 31752052.
- ^ Thal SC, Schaible EV, Neuhaus W, Scheffer D, Brandstetter M, Engelhard K, et al. (May 2013). "Inhibition of proteasomal glucocorticoid receptor degradation restores dexamethasone-mediated stabilization of the blood-brain barrier after traumatic brain injury". Critical Care Medicine. 41 (5): 1305–1315. doi:10.1097/CCM.0b013e31827ca494. PMID 23474678. S2CID 23399305.
- ^ Roberts I, Sydenham E (December 2012). "Barbiturates for acute traumatic brain injury". The Cochrane Database of Systematic Reviews. 2012 (12): CD000033. doi:10.1002/14651858.CD000033.pub2. PMC 7061245. PMID 23235573.
- ^ Arango MF, Bainbridge D (October 2008). "Magnesium for acute traumatic brain injury". The Cochrane Database of Systematic Reviews (4): CD005400. doi:10.1002/14651858.CD005400.pub3. PMID 18843689.
- ^ Langham J, Goldfrad C, Teasdale G, Shaw D, Rowan K (2003). "Calcium channel blockers for acute traumatic brain injury". The Cochrane Database of Systematic Reviews (4): CD000565. doi:10.1002/14651858.CD000565. PMC 7032539. PMID 14583925.
- ^ Lewis SR, Baker PE, Andrews PJ, Cheng A, Deol K, Hammond N, Saxena M (October 2020). "Interventions to reduce body temperature to 35 °C to 37 °C in adults and children with traumatic brain injury". The Cochrane Database of Systematic Reviews. 2020 (10): CD006811. doi:10.1002/14651858.CD006811.pub4. PMC 8094748. PMID 33126293.
- ^ a b Watson HI, Shepherd AA, Rhodes JK, Andrews PJ (June 2018). "Revisited: A Systematic Review of Therapeutic Hypothermia for Adult Patients Following Traumatic Brain Injury". Critical Care Medicine. 46 (6): 972–979. doi:10.1097/CCM.0000000000003125. hdl:20.500.11820/fae8c4d4-8286-42db-b08d-1369e9092e06. PMID 29601315. S2CID 4495428.
- ^ Crompton EM, Lubomirova I, Cotlarciuc I, Han TS, Sharma SD, Sharma P (April 2017). "Meta-Analysis of Therapeutic Hypothermia for Traumatic Brain Injury in Adult and Pediatric Patients". Critical Care Medicine. 45 (4): 575–583. doi:10.1097/CCM.0000000000002205. PMID 27941370. S2CID 19064475.
- ^ Lewis SR, Evans DJ, Butler AR, Schofield-Robinson OJ, Alderson P (September 2017). "Hypothermia for traumatic brain injury". The Cochrane Database of Systematic Reviews. 2017 (9): CD001048. doi:10.1002/14651858.CD001048.pub5. PMC 6483736. PMID 28933514.
- ^ Nilsson P, Hillered L, Pontén U, Ungerstedt U (September 1990). "Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats". Journal of Cerebral Blood Flow and Metabolism. 10 (5): 631–637. doi:10.1038/jcbfm.1990.115. PMID 2384536.
- ^ Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, et al. (June 2005). "Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study". Journal of Cerebral Blood Flow and Metabolism. 25 (6): 763–774. doi:10.1038/sj.jcbfm.9600073. PMC 4347944. PMID 15716852.
- ^ Dengler J, Frenzel C, Vajkoczy P, Wolf S, Horn P (November 2011). "Cerebral tissue oxygenation measured by two different probes: challenges and interpretation". Intensive Care Medicine. 37 (11): 1809–1815. doi:10.1007/s00134-011-2316-z. PMID 21809108. S2CID 23154956.
- ^ Rosenthal G, Furmanov A, Itshayek E, Shoshan Y, Singh V (April 2014). "Assessment of a noninvasive cerebral oxygenation monitor in patients with severe traumatic brain injury". Journal of Neurosurgery. 120 (4): 901–907. doi:10.3171/2013.12.JNS131089. PMID 24484228.
- ^ Yates D, Aktar R, Hill J (October 2007). "Assessment, investigation, and early management of head injury: summary of NICE guidance". BMJ. 335 (7622): 719–720. doi:10.1136/bmj.39331.702951.47. PMC 2001047. PMID 17916856.
- ^ a b c Bennett MH, Trytko B, Jonker B (December 2012). "Hyperbaric oxygen therapy for the adjunctive treatment of traumatic brain injury". The Cochrane Database of Systematic Reviews. 12: CD004609. doi:10.1002/14651858.CD004609.pub3. PMC 11561354. PMID 23235612.
- ^ Rockswold SB, Rockswold GL, Defillo A (March 2007). "Hyperbaric oxygen in traumatic brain injury". Neurological Research. 29 (2): 162–172. doi:10.1179/016164107X181798. PMID 17439701. S2CID 35914918.
- ^ Gertler P, Tate RL, Cameron ID (December 2015). "Non-pharmacological interventions for depression in adults and children with traumatic brain injury". The Cochrane Database of Systematic Reviews. 2015 (12): CD009871. doi:10.1002/14651858.CD009871.pub2. PMC 8761477. PMID 26663136.
- ^ Maruta J, Lee SW, Jacobs EF, Ghajar J (October 2010). "A unified science of concussion". Annals of the New York Academy of Sciences. 1208 (1): 58–66. Bibcode:2010NYASA1208...58M. doi:10.1111/j.1749-6632.2010.05695.x. PMC 3021720. PMID 20955326.
- ^ Copplestone S, Welbourne J (February 2018). "A narrative review of the clinical application of pressure reactiviy indices in the neurocritical care unit". British Journal of Neurosurgery. 32 (1): 4–12. doi:10.1080/02688697.2017.1416063. PMID 29298527. S2CID 26020481.
- ^ Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. (December 2012). "A trial of intracranial-pressure monitoring in traumatic brain injury". The New England Journal of Medicine. 367 (26): 2471–2481. doi:10.1056/NEJMoa1207363. PMC 3565432. PMID 23234472.
- ^ Carron SF, Alwis DS, Rajan R (2016). "Traumatic Brain Injury and Neuronal Functionality Changes in Sensory Cortex". Frontiers in Systems Neuroscience. 10: 47. doi:10.3389/fnsys.2016.00047. PMC 4889613. PMID 27313514.
- ^ Alwis DS, Yan EB, Morganti-Kossmann MC, Rajan R (2012). "Sensory cortex underpinnings of traumatic brain injury deficits". PLOS ONE. 7 (12): e52169. Bibcode:2012PLoSO...752169A. doi:10.1371/journal.pone.0052169. PMC 3528746. PMID 23284921.
- ^ Johnstone VP, Shultz SR, Yan EB, O'Brien TJ, Rajan R (November 2014). "The acute phase of mild traumatic brain injury is characterized by a distance-dependent neuronal hypoactivity". Journal of Neurotrauma. 31 (22): 1881–1895. doi:10.1089/neu.2014.3343. PMC 4224042. PMID 24927383.
- ^ Burns TF (2019). Burns (2019): Temporal neuronal activity patterns in barrel cortex to simple and complex stimuli and the effects of traumatic brain injury. Monash University. Thesis. 10.26180/5b7166ad13e47 (thesis). Monash University. doi:10.26180/5b7166ad13e47.
Cited texts
- Boake C, Diller L (2005). "History of rehabilitation for traumatic brain injury". In High WM, Sander AM, Struchen MA, Hart KA (eds.). Rehabilitation for Traumatic Brain Injury. Oxford [Oxfordshire]: Oxford University Press. ISBN 978-0-19-517355-0.
- Granacher RA (2007). Traumatic Brain Injury: Methods for Clinical & Forensic Neuropsychiatric Assessment, Second Edition. Boca Raton: CRC. ISBN 978-0-8493-8138-6.
- LaPlaca MC, Simon CM, Prado GR, Cullen DR (2007). "CNS injury biomechanics and experimental models". In Weber JT (ed.). Neurotrauma: New Insights Into Pathology and Treatment. Amsterdam: Academic Press. ISBN 978-0-444-53017-2.
- Marion DW (1999). "Introduction". In Marion DW (ed.). Traumatic Brain Injury. Stuttgart: Thieme. ISBN 978-0-86577-727-9.
The original version of this article contained text from the NINDS public domain pages on TBI Archived December 18, 2016, at the Wayback Machine
External links
- The Brain Injury Hub – information and practical advice to parents and family members of children with acquired brain injury Archived October 5, 2020, at the Wayback Machine
- Defense and Veterans Brain Injury Center – U.S. Department of Defense Military Health System center for traumatic brain injury Archived October 12, 2019, at the Wayback Machine
