Antimicrobial resistance

Antimicrobial resistance (AMR or AR) occurs when microbes evolve mechanisms that protect them from antimicrobials, which are drugs used to treat infections.[2] This resistance affects all classes of microbes, including bacteria (antibiotic resistance), viruses (antiviral resistance), protozoa (antiprotozoal resistance), and fungi (antifungal resistance). Together, these adaptations fall under the AMR umbrella, posing significant challenges to healthcare worldwide.[3] Misuse and improper management of antimicrobials are primary drivers of this resistance, though it can also occur naturally through genetic mutations and the spread of resistant genes.[4]
Microbes resistant to multiple drugs are termed multidrug-resistant (MDR) and are sometimes called superbugs.[5] Antibiotic resistance, a significant AMR subset, enables bacteria to survive antibiotic treatment, complicating infection management and treatment options.[3] Resistance arises through spontaneous mutation, horizontal gene transfer, and increased selective pressure from antibiotic overuse, both in medicine and agriculture, which accelerates resistance development.[6]
The burden of AMR is immense, with nearly 5 million annual deaths associated with resistant infections.[7] Infections from AMR microbes are more challenging to treat and often require costly alternative therapies that may have more severe side effects.[8] Preventive measures, such as using narrow-spectrum antibiotics and improving hygiene practices, aim to reduce the spread of resistance.[9]
The WHO claims that AMR is one of the top global public health and development threats, estimating that bacterial AMR was directly responsible for 1.27 million global deaths in 2019 and contributed to 4.95 million deaths[10]. Moreover, the WHO and other international bodies warn that AMR could lead to up to 10 million deaths annually by 2050 unless actions are taken.[11] Global initiatives, such as calls for international AMR treaties, emphasize coordinated efforts to limit misuse, fund research, and provide access to necessary antimicrobials in developing nations. However, the COVID-19 pandemic redirected resources and scientific attention away from AMR, intensifying the challenge.[12]
Definition
[edit]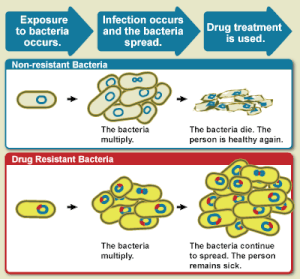
The WHO defines antimicrobial resistance as a microorganism's resistance to an antimicrobial drug that was once able to treat an infection by that microorganism.[3] A person cannot become resistant to antibiotics. Resistance is a property of the microbe, not a person or other organism infected by a microbe.[14] All types of microbes can develop drug resistance. Thus, there are antibiotic, antifungal, antiviral and antiparasitic resistance.[4][8]
Antibiotic resistance is a subset of antimicrobial resistance. This more specific resistance is linked to bacteria and thus broken down into two further subsets, microbiological and clinical. Microbiological resistance is the most common and occurs from genes, mutated or inherited, that allow the bacteria to resist the mechanism to kill the microbe associated with certain antibiotics. Clinical resistance is shown through the failure of many therapeutic techniques where the bacteria that are normally susceptible to a treatment become resistant after surviving the outcome of the treatment. In both cases of acquired resistance, the bacteria can pass the genetic catalyst for resistance through horizontal gene transfer: conjugation, transduction, or transformation. This allows the resistance to spread across the same species of pathogen or even similar bacterial pathogens.[15]
Overview
[edit]WHO report released April 2014 stated, "this serious threat is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country. Antibiotic resistance—when bacteria change so antibiotics no longer work in people who need them to treat infections—is now a major threat to public health."[16]
Each year, nearly 5 million deaths are associated with AMR globally.[7] In 2019, global deaths attributable to AMR numbered 1.27 million in 2019. That same year, AMR may have contributed to 5 million deaths and one in five people who died due to AMR were children under five years old.[17]
In 2018, WHO considered antibiotic resistance to be one of the biggest threats to global health, food security and development.[18] Deaths attributable to AMR vary by area:
| Place | Deaths per 100,000 attributable to AMR[17] |
|---|---|
| North Africa and Middle East | 11.2 |
| Southeast and East Asia, and Oceania | 11.7 |
| Latin America and Caribbean | 14.4 |
| Central and Eastern Europe and Central Asia | 17.6 |
| South Asia | 21.5 |
| Sub-Saharan Africa | 23.7 |
The European Centre for Disease Prevention and Control calculated that in 2015 there were 671,689 infections in the EU and European Economic Area caused by antibiotic-resistant bacteria, resulting in 33,110 deaths. Most were acquired in healthcare settings.[19][20] In 2019 there were 133,000 deaths caused by AMR.[21]
Causes
[edit]AMR is driven largely by the misuse and overuse of antimicrobials.[7] Yet, at the same time, many people around the world do not have access to essential antimicrobials.[7] This leads to microbes either evolving a defense against drugs used to treat them, or certain strains of microbes that have a natural resistance to antimicrobials becoming much more prevalent than the ones that are easily defeated with medication.[22] While antimicrobial resistance does occur naturally over time, the use of antimicrobial agents in a variety of settings both within the healthcare industry and outside of has led to antimicrobial resistance becoming increasingly more prevalent.[23]
Although many microbes develop resistance to antibiotics over time though natural mutation, overprescribing and inappropriate prescription of antibiotics have accelerated the problem. It is possible that as many as 1 in 3 prescriptions written for antibiotics are unnecessary.[24] Every year, approximately 154 million prescriptions for antibiotics are written. Of these, up to 46 million are unnecessary or inappropriate for the condition that the patient has.[24] Microbes may naturally develop resistance through genetic mutations that occur during cell division, and although random mutations are rare, many microbes reproduce frequently and rapidly, increasing the chances of members of the population acquiring a mutation that increases resistance.[25] Many individuals stop taking antibiotics when they begin to feel better. When this occurs, it is possible that the microbes that are less susceptible to treatment still remain in the body. If these microbes are able to continue to reproduce, this can lead to an infection by bacteria that are less susceptible or even resistant to an antibiotic.[25]
Natural occurrence
[edit]
AMR is a naturally occurring process.[2] Antimicrobial resistance can evolve naturally due to continued exposure to antimicrobials. Natural selection means that organisms that are able to adapt to their environment, survive, and continue to produce offspring.[26] As a result, the types of microorganisms that are able to survive over time with continued attack by certain antimicrobial agents will naturally become more prevalent in the environment, and those without this resistance will become obsolete.[23]
Some contemporary antimicrobial resistances have also evolved naturally before the use of antimicrobials of human clinical uses. For instance, methicillin-resistance evolved as a pathogen of hedgehogs, possibly as a co-evolutionary adaptation of the pathogen to hedgehogs that are infected by a dermatophyte that naturally produces antibiotics.[27] Also, many soil fungi and bacteria are natural competitors and the original antibiotic penicillin discovered by Alexander Fleming rapidly lost clinical effectiveness in treating humans and, furthermore, none of the other natural penicillins (F, K, N, X, O, U1 or U6) are currently in clinical use.[citation needed]
Antimicrobial resistance can be acquired from other microbes through swapping genes in a process termed horizontal gene transfer. This means that once a gene for resistance to an antibiotic appears in a microbial community, it can then spread to other microbes in the community, potentially moving from a non-disease causing microbe to a disease-causing microbe. This process is heavily driven by the natural selection processes that happen during antibiotic use or misuse.[28]
Over time, most of the strains of bacteria and infections present will be the type resistant to the antimicrobial agent being used to treat them, making this agent now ineffective to defeat most microbes. With the increased use of antimicrobial agents, there is a speeding up of this natural process.[29]
Self-medication
[edit]In the vast majority of countries, antibiotics can only be prescribed by a doctor and supplied by a pharmacy.[30] Self-medication by consumers is defined as "the taking of medicines on one's own initiative or on another person's suggestion, who is not a certified medical professional", and it has been identified as one of the primary reasons for the evolution of antimicrobial resistance.[31] Self-medication with antibiotics is an unsuitable way of using them but a common practice in resource-constrained countries. The practice exposes individuals to the risk of bacteria that have developed antimicrobial resistance.[32] Many people resort to this out of necessity, when access to a physician is unavailable, or when patients have a limited amount of time or money to see a doctor.[33] This increased access makes it extremely easy to obtain antimicrobials. An example is India, where in the state of Punjab 73% of the population resorted to treating their minor health issues and chronic illnesses through self-medication.[31]
Self-medication is higher outside the hospital environment, and this is linked to higher use of antibiotics, with the majority of antibiotics being used in the community rather than hospitals. The prevalence of self-medication in low- and middle-income countries (LMICs) ranges from 8.1% to 93%. Accessibility, affordability, and conditions of health facilities, as well as the health-seeking behavior, are factors that influence self-medication in low- and middle-income countries.[32] Two significant issues with self-medication are the lack of knowledge of the public on, firstly, the dangerous effects of certain antimicrobials (for example ciprofloxacin which can cause tendonitis, tendon rupture and aortic dissection)[34][35] and, secondly, broad microbial resistance and when to seek medical care if the infection is not clearing. In order to determine the public's knowledge and preconceived notions on antibiotic resistance, a screening of 3,537 articles published in Europe, Asia, and North America was done. Of the 55,225 total people surveyed in the articles, 70% had heard of antibiotic resistance previously, but 88% of those people thought it referred to some type of physical change in the human body.[31]
Clinical misuse
[edit]Clinical misuse by healthcare professionals is another contributor to increased antimicrobial resistance. Studies done in the US show that the indication for treatment of antibiotics, choice of the agent used, and the duration of therapy was incorrect in up to 50% of the cases studied.[36] In 2010 and 2011 about a third of antibiotic prescriptions in outpatient settings in the United States were not necessary.[37] Another study in an intensive care unit in a major hospital in France has shown that 30% to 60% of prescribed antibiotics were unnecessary.[36] These inappropriate uses of antimicrobial agents promote the evolution of antimicrobial resistance by supporting the bacteria in developing genetic alterations that lead to resistance.[38]
According to research conducted in the US that aimed to evaluate physicians' attitudes and knowledge on antimicrobial resistance in ambulatory settings, only 63% of those surveyed reported antibiotic resistance as a problem in their local practices, while 23% reported the aggressive prescription of antibiotics as necessary to avoid failing to provide adequate care.[39] This demonstrates how a majority of doctors underestimate the impact that their own prescribing habits have on antimicrobial resistance as a whole. It also confirms that some physicians may be overly cautious and prescribe antibiotics for both medical or legal reasons, even when clinical indications for use of these medications are not always confirmed. This can lead to unnecessary antimicrobial use, a pattern which may have worsened during the COVID-19 pandemic.[40][41]
Studies have shown that common misconceptions about the effectiveness and necessity of antibiotics to treat common mild illnesses contribute to their overuse.[42][43]
Important to the conversation of antibiotic use is the veterinary medical system. Veterinary oversight is required by law for all medically important antibiotics.[44] Veterinarians use the Pharmacokinetic/pharmacodynamic model (PK/PD) approach to ensuring that the correct dose of the drug is delivered to the correct place at the correct timing.[45]
Pandemics, disinfectants and healthcare systems
[edit]Increased antibiotic use during the early waves of the COVID-19 pandemic may exacerbate this global health challenge.[46][47] Moreover, pandemic burdens on some healthcare systems may contribute to antibiotic-resistant infections.[48] On the other hand, "increased hand hygiene, decreased international travel, and decreased elective hospital procedures may have reduced AMR pathogen selection and spread in the short term" during the COVID-19 pandemic.[49] The use of disinfectants such as alcohol-based hand sanitizers, and antiseptic hand wash may also have the potential to increase antimicrobial resistance.[50] Extensive use of disinfectants can lead to mutations that induce antimicrobial resistance.[51]
A 2024 United Nations High-Level Meeting on AMR has pledged to reduce deaths associated with bacterial AMR by 10% over the next six years.[7][52] In their first major declaration on the issue since 2016, global leaders also committed to raising $100 million to update and implement AMR action plans.[53] However, the final draft of the declaration omitted an earlier target to reduce antibiotic use in animals by 30% by 2030, due to opposition from meat-producing countries and the farming industry. Critics argue this omission is a major weakness, as livestock accounts for around 73% of global sales of antimicrobial agents, including antibiotics, antivirals, and antiparasitics.
Environmental pollution
[edit]Considering the complex interactions between humans, animals and the environment, it is also important to consider the environmental aspects and contributors to antimicrobial resistance.[54] Although there are still some knowledge gaps in understanding the mechanisms and transmission pathways,[55] environmental pollution is considered a significant contributor to antimicrobial resistance.[56] Important contributing factors are through "antibiotic residues", "industrial effluents", " agricultural runoffs", "heavy metals", "biocides and pesticides" and "sewage and wastewater" that create reservoirs for resistant genes and bacteria that facilitates the transfer of human pathogens.[55][56] Unused or expired antibiotics, if not disposed of properly, can enter water systems and soil.[56] Discharge from pharmaceutical manufacturing and other industrial companies can also introduce antibiotics and other chemicals into the environment.[56] These factors allow for creating selective pressure for resistant bacteria.[56] Antibiotics used in livestock and aquaculture can contaminate soil and water, which promotes resistance in environmental microbes.[55] Heavy metals such as zinc, copper and mercury, and also biocides and pesticides, can co- select for antibiotic resistance,[56] enhancing their speed.[55] Inadequate treatment of sewage and wastewater allows resistant bacteria and genes to spread through water systems.[55][55]
Food production
[edit]Livestock
[edit]
The antimicrobial resistance crisis also extends to the food industry, specifically with food producing animals. With an ever-increasing human population, there is constant pressure to intensify productivity in many agricultural sectors, including the production of meat as a source of protein.[57] Antibiotics are fed to livestock to act as growth supplements, and a preventive measure to decrease the likelihood of infections.[58]
Farmers typically use antibiotics in animal feed to improve growth rates and prevent infections. However, this is illogical as antibiotics are used to treat infections and not prevent infections. 80% of antibiotic use in the U.S. is for agricultural purposes and about 70% of these are medically important.[59] Overusing antibiotics gives the bacteria time to adapt leaving higher doses or even stronger antibiotics needed to combat the infection. Though antibiotics for growth promotion were banned throughout the EU in 2006, 40 countries worldwide still use antibiotics to promote growth.[60]
This can result in the transfer of resistant bacterial strains into the food that humans eat, causing potentially fatal transfer of disease. While the practice of using antibiotics as growth promoters does result in better yields and meat products, it is a major issue and needs to be decreased in order to prevent antimicrobial resistance.[61] Though the evidence linking antimicrobial usage in livestock to antimicrobial resistance is limited, the World Health Organization Advisory Group on Integrated Surveillance of Antimicrobial Resistance strongly recommended the reduction of use of medically important antimicrobials in livestock. Additionally, the Advisory Group stated that such antimicrobials should be expressly prohibited for both growth promotion and disease prevention in food producing animals.[62]
By mapping antimicrobial consumption in livestock globally, it was predicted that in 228 countries there would be a total 67% increase in consumption of antibiotics by livestock by 2030. In some countries such as Brazil, Russia, India, China, and South Africa it is predicted that a 99% increase will occur.[29] Several countries have restricted the use of antibiotics in livestock, including Canada, China, Japan, and the US. These restrictions are sometimes associated with a reduction of the prevalence of antimicrobial resistance in humans.[62]
In the United States the Veterinary Feed Directive went into practice in 2017 dictating that All medically important antibiotics to be used in feed or water for food animal species require a veterinary feed directive (VFD) or a prescription.[63]
Pesticides
[edit]Most pesticides protect crops against insects and plants, but in some cases antimicrobial pesticides are used to protect against various microorganisms such as bacteria, viruses, fungi, algae, and protozoa. The overuse of many pesticides in an effort to have a higher yield of crops has resulted in many of these microbes evolving a tolerance against these antimicrobial agents. Currently there are over 4000 antimicrobial pesticides registered with the US Environmental Protection Agency (EPA) and sold to market, showing the widespread use of these agents.[64] It is estimated that for every single meal a person consumes, 0.3 g of pesticides is used, as 90% of all pesticide use is in agriculture. A majority of these products are used to help defend against the spread of infectious diseases, and hopefully protect public health. But out of the large amount of pesticides used, it is also estimated that less than 0.1% of those antimicrobial agents, actually reach their targets. That leaves over 99% of all pesticides used available to contaminate other resources.[65] In soil, air, and water these antimicrobial agents are able to spread, coming in contact with more microorganisms and leading to these microbes evolving mechanisms to tolerate and further resist pesticides. The use of antifungal azole pesticides that drive environmental azole resistance have been linked to azole resistance cases in the clinical setting.[66] The same issues confront the novel antifungal classes (e.g. orotomides) which are again being used in both the clinic and agriculture.[67]
Wild birds
[edit]Wildlife, including wild and migratory birds, serve as a reservoir for zoonotic disease and antimicrobial-resistant organisms. Birds are a key link between the transmission of zoonotic diseases to human populations. By the same token, increased contact between wild birds and human populations (including domesticated animals), has increased the amount of anti-microbial resistance (AMR) to the bird population.[6] The introduction of AMR to wild birds positively correlates with human pollution and increased human contact. Additionally, wild birds can participate in horizontal gene transfer with bacteria, leading to the transmission of antibiotic-resistant genes (ARG).[26]
For simplicity, wild bird populations can be divided into two major categories, wild sedentary birds and wild migrating birds. Wild sedentary bird exposure to AMR is through increased contact with densely populated areas, human waste, domestic animals, and domestic animal/livestock waste. Wild migrating birds interact with sedentary birds in different environments along their migration route. This increases the rate and diversity of AMR across varying ecosystems.[6]
Neglect of wildlife in the global discussions surrounding health security and AMR, creates large barriers to true AMR surveillance. The surveillance of anti-microbial resistant organisms in wild birds is a potential metric for the rate of AMR in the environment. This surveillance also allows for further investigation into the transmission routs between different ecosystems and human populations (including domesticated animals and livestock).[6] Such information gathered from wild bird biomes, can help identify patterns of diseased transmission and better target interventions. These targeted interventions can inform the use of antimicrobial agents and reduce the persistence of multi-drug resistant organisms.[18][27]
Gene transfer from ancient microorganisms
[edit]
Permafrost is a term used to refer to any ground that remained frozen for two years or more, with the oldest known examples continuously frozen for around 700,000 years.[69] In the recent decades, permafrost has been rapidly thawing due to climate change.[70]: 1237 The cold preserves any organic matter inside the permafrost, and it is possible for microorganisms to resume their life functions once it thaws. While some common pathogens such as influenza, smallpox or the bacteria associated with pneumonia have failed to survive intentional attempts to revive them,[71] more cold-adapted microorganisms such as anthrax, or several ancient plant and amoeba viruses, have successfully survived prolonged thaw.[72][73][74][75][76]
Some scientists have argued that the inability of known causative agents of contagious diseases to survive being frozen and thawed makes this threat unlikely. Instead, there have been suggestions that when modern pathogenic bacteria interact with the ancient ones, they may, through horizontal gene transfer, pick up genetic sequences which are associated with antimicrobial resistance, exacerbating an already difficult issue.[77] Antibiotics to which permafrost bacteria have displayed at least some resistance include chloramphenicol, streptomycin, kanamycin, gentamicin, tetracycline, spectinomycin and neomycin.[78] However, other studies show that resistance levels in ancient bacteria to modern antibiotics remain lower than in the contemporary bacteria from the active layer of thawed ground above them,[68] which may mean that this risk is "no greater" than from any other soil.[79]
Prevention
[edit]
There have been increasing public calls for global collective action to address the threat, including a proposal for an international treaty on antimicrobial resistance. Further detail and attention is still needed in order to recognize and measure trends in resistance on the international level; the idea of a global tracking system has been suggested but implementation has yet to occur. A system of this nature would provide insight to areas of high resistance as well as information necessary for evaluating programs, introducing interventions and other changes made to fight or reverse antibiotic resistance.[80][81]
Duration of antimicrobials
[edit]Delaying or minimizing the use of antibiotics for certain conditions may help safely reduce their use.[82] Antimicrobial treatment duration should be based on the infection and other health problems a person may have.[83] For many infections once a person has improved there is little evidence that stopping treatment causes more resistance.[83] Some, therefore, feel that stopping early may be reasonable in some cases.[83] Other infections, however, do require long courses regardless of whether a person feels better.[83]
Delaying antibiotics for ailments such as a sore throat and otitis media may have not different in the rate of complications compared with immediate antibiotics, for example.[82] When treating respiratory tract infections, clinical judgement is required as to the appropriate treatment (delayed or immediate antibiotic use).[82]
The study, "Shorter and Longer Antibiotic Durations for Respiratory Infections: To Fight Antimicrobial Resistance—A Retrospective Cross-Sectional Study in a Secondary Care Setting in the UK," highlights the urgency of reevaluating antibiotic treatment durations amidst the global challenge of antimicrobial resistance (AMR). It investigates the effectiveness of shorter versus longer antibiotic regimens for respiratory tract infections (RTIs) in a UK secondary care setting, emphasizing the need for evidence-based prescribing practices to optimize patient outcomes and combat AMR.[84]
Monitoring and mapping
[edit]There are multiple national and international monitoring programs for drug-resistant threats, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant S. aureus (VRSA), extended spectrum beta-lactamase (ESBL) producing Enterobacterales, vancomycin-resistant Enterococcus (VRE), and multidrug-resistant Acinetobacter baumannii (MRAB).[85]
ResistanceOpen is an online global map of antimicrobial resistance developed by HealthMap which displays aggregated data on antimicrobial resistance from publicly available and user submitted data.[86][87] The website can display data for a 25 miles (40 km) radius from a location. Users may submit data from antibiograms for individual hospitals or laboratories. European data is from the EARS-Net (European Antimicrobial Resistance Surveillance Network), part of the ECDC. ResistanceMap is a website by the Center for Disease Dynamics, Economics & Policy and provides data on antimicrobial resistance on a global level.[88]
The WHO's AMR global action plan also recommends antimicrobial resistance surveillance in animals.[89] Initial steps in the EU for establishing the veterinary counterpart EARS-Vet (EARS-Net for veterinary medicine) have been made.[90] AMR data from pets in particular is scarce, but needed to support antibiotic stewardship in veterinary medicine.[91]
By comparison there is a lack of national and international monitoring programs for antifungal resistance.[92]
Limiting antimicrobial use in humans
[edit]Antimicrobial stewardship programmes appear useful in reducing rates of antimicrobial resistance.[93] The antimicrobial stewardship program will also provide pharmacists with the knowledge to educate patients that antibiotics will not work for a virus for example.[94]
Excessive antimicrobial use has become one of the top contributors to the evolution of antimicrobial resistance. Since the beginning of the antimicrobial era, antimicrobials have been used to treat a wide range of infectious diseases.[95] Overuse of antimicrobials has become the primary cause of rising levels of antimicrobial resistance. The main problem is that doctors are willing to prescribe antimicrobials to ill-informed individuals who believe that antimicrobials can cure nearly all illnesses, including viral infections like the common cold. In an analysis of drug prescriptions, 36% of individuals with a cold or an upper respiratory infection (both usually viral in origin) were given prescriptions for antibiotics.[96] These prescriptions accomplished nothing other than increasing the risk of further evolution of antibiotic resistant bacteria.[97] Using antimicrobials without prescription is another driving force leading to the overuse of antibiotics to self-treat diseases like the common cold, cough, fever, and dysentery resulting in an epidemic of antibiotic resistance in countries like Bangladesh, risking its spread around the globe.[98] Introducing strict antibiotic stewardship in the outpatient setting to reduce inappropriate prescribing of antibiotics may reduce the emerging bacterial resistance.[99]
The WHO AWaRe (Access, Watch, Reserve) guidance and antibiotic book has been introduced to guide antibiotic choice for the 30 most common infections in adults and children to reduce inappropriate prescribing in primary care and hospitals. Narrow-spectrum antibiotics are preferred due to their lower resistance potential, and broad-spectrum antibiotics are only recommended for people with more severe symptoms. Some antibiotics are more likely to confer resistance, so are kept as reserve antibiotics in the AWaRe book.[100]
Various diagnostic strategies have been employed to prevent the overuse of antifungal therapy in the clinic, proving a safe alternative to empirical antifungal therapy, and thus underpinning antifungal stewardship schemes.[101]
At the hospital level
[edit]Antimicrobial stewardship teams in hospitals are encouraging optimal use of antimicrobials.[102] The goals of antimicrobial stewardship are to help practitioners pick the right drug at the right dose and duration of therapy while preventing misuse and minimizing the development of resistance. Stewardship interventions may reduce the length of stay by an average of slightly over 1 day while not increasing the risk of death.[103] Dispensing, to discharged in-house patients, the exact number of antibiotic pharmaceutical units necessary to complete an ongoing treatment can reduce antibiotic leftovers within the community as community pharmacies can have antibiotic package inefficiencies.[104]
At the primary care level
[edit]Given the volume of care provided in primary care (general practice), recent strategies have focused on reducing unnecessary antimicrobial prescribing in this setting. Simple interventions, such as written information explaining when taking antibiotics is not necessary, for example in common infections of the upper respiratory tract, have been shown to reduce antibiotic prescribing.[105] Various tools are also available to help professionals decide if prescribing antimicrobials is necessary.
Parental expectations, driven by the worry for their children's health, can influence how often children are prescribed antibiotics. Parents often rely on their clinician for advice and reassurance. However a lack of plain language information and not having adequate time for consultation negatively impacts this relationship. In effect parents often rely on past experiences in their expectations rather than reassurance from the clinician. Adequate time for consultation and plain language information can help parents make informed decisions and avoid unnecessary antibiotic use.[106]
The prescriber should closely adhere to the five rights of drug administration: the right patient, the right drug, the right dose, the right route, and the right time.[107] Microbiological samples should be taken for culture and sensitivity testing before treatment when indicated and treatment potentially changed based on the susceptibility report.[108][109]
Health workers and pharmacists can help tackle antibiotic resistance by: enhancing infection prevention and control; only prescribing and dispensing antibiotics when they are truly needed; prescribing and dispensing the right antibiotic(s) to treat the illness.[16] A unit dose system implemented in community pharmacies can also reduce antibiotic leftovers at households.[104]
At the individual level
[edit]People can help tackle resistance by using antibiotics only when infected with a bacterial infection and prescribed by a doctor; completing the full prescription even if the user is feeling better, never sharing antibiotics with others, or using leftover prescriptions.[16] Taking antibiotics when not needed won't help the user, but instead give bacteria the option to adapt and leave the user with the side effects that come with the certain type of antibiotic.[110] The CDC recommends that you follow these behaviors so that you avoid these negative side effects and keep the community safe from spreading drug-resistant bacteria.[110] Practicing basic bacterial infection prevention courses, such as hygiene, also helps to prevent the spread of antibiotic-resistant bacteria.[111]
Country examples
[edit]- The Netherlands has the lowest rate of antibiotic prescribing in the OECD, at a rate of 11.4 defined daily doses (DDD) per 1,000 people per day in 2011. The defined daily dose (DDD) is a statistical measure of drug consumption, defined by the World Health Organization (WHO).[112]
- Germany and Sweden also have lower prescribing rates, with Sweden's rate having been declining since 2007.
- Greece, France and Belgium have high prescribing rates for antibiotics of more than 28 DDD.[113]
Water, sanitation, hygiene
[edit]Infectious disease control through improved water, sanitation and hygiene (WASH) infrastructure needs to be included in the antimicrobial resistance (AMR) agenda. The "Interagency Coordination Group on Antimicrobial Resistance" stated in 2018 that "the spread of pathogens through unsafe water results in a high burden of gastrointestinal disease, increasing even further the need for antibiotic treatment."[114] This is particularly a problem in developing countries where the spread of infectious diseases caused by inadequate WASH standards is a major driver of antibiotic demand.[115] Growing usage of antibiotics together with persistent infectious disease levels have led to a dangerous cycle in which reliance on antimicrobials increases while the efficacy of drugs diminishes.[115] The proper use of infrastructure for water, sanitation and hygiene (WASH) can result in a 47–72 percent decrease of diarrhea cases treated with antibiotics depending on the type of intervention and its effectiveness.[115] A reduction of the diarrhea disease burden through improved infrastructure would result in large decreases in the number of diarrhea cases treated with antibiotics. This was estimated as ranging from 5 million in Brazil to up to 590 million in India by the year 2030.[115] The strong link between increased consumption and resistance indicates that this will directly mitigate the accelerating spread of AMR.[115] Sanitation and water for all by 2030 is Goal Number 6 of the Sustainable Development Goals.[116]
An increase in hand washing compliance by hospital staff results in decreased rates of resistant organisms.[117]
Water supply and sanitation infrastructure in health facilities offer significant co-benefits for combatting AMR, and investment should be increased.[114] There is much room for improvement: WHO and UNICEF estimated in 2015 that globally 38% of health facilities did not have a source of water, nearly 19% had no toilets and 35% had no water and soap or alcohol-based hand rub for handwashing.[118]
Industrial wastewater treatment
[edit]Manufacturers of antimicrobials need to improve the treatment of their wastewater (by using industrial wastewater treatment processes) to reduce the release of residues into the environment.[114]
Limiting antimicrobial use in animals and farming
[edit]It is established that the use of antibiotics in animal husbandry can give rise to AMR resistances in bacteria found in food animals to the antibiotics being administered (through injections or medicated feeds).[119] For this reason only antimicrobials that are deemed "not-clinically relevant" are used in these practices.
Unlike resistance to antibacterials, antifungal resistance can be driven by arable farming, currently there is no regulation on the use of similar antifungal classes in agriculture and the clinic.[92][67]
Recent studies have shown that the prophylactic use of "non-priority" or "non-clinically relevant" antimicrobials in feeds can potentially, under certain conditions, lead to co-selection of environmental AMR bacteria with resistance to medically important antibiotics.[120] The possibility for co-selection of AMR resistances in the food chain pipeline may have far-reaching implications for human health.[120][121]
Country examples
[edit]Europe
[edit]In 1997, European Union health ministers voted to ban avoparcin and four additional antibiotics used to promote animal growth in 1999.[122] In 2006 a ban on the use of antibiotics in European feed, with the exception of two antibiotics in poultry feeds, became effective.[123] In Scandinavia, there is evidence that the ban has led to a lower prevalence of antibiotic resistance in (nonhazardous) animal bacterial populations.[124] As of 2004, several European countries established a decline of antimicrobial resistance in humans through limiting the use of antimicrobials in agriculture and food industries without jeopardizing animal health or economic cost.[125]
United States
[edit]The United States Department of Agriculture (USDA) and the Food and Drug Administration (FDA) collect data on antibiotic use in humans and in a more limited fashion in animals.[126] About 80% of antibiotic use in the U.S. is for agriculture purposes, and about 70% of these are medically important.[59] This gives reason for concern about the antibiotic resistance crisis in the U.S. and more reason to monitor it. The FDA first determined in 1977 that there is evidence of emergence of antibiotic-resistant bacterial strains in livestock. The long-established practice of permitting OTC sales of antibiotics (including penicillin and other drugs) to lay animal owners for administration to their own animals nonetheless continued in all states. In 2000, the FDA announced their intention to revoke approval of fluoroquinolone use in poultry production because of substantial evidence linking it to the emergence of fluoroquinolone-resistant Campylobacter infections in humans. Legal challenges from the food animal and pharmaceutical industries delayed the final decision to do so until 2006.[127] Fluroquinolones have been banned from extra-label use in food animals in the USA since 2007.[128] However, they remain widely used in companion and exotic animals.[129]
Global action plans and awareness
[edit]At the 79th United Nations General Assembly High-Level Meeting on AMR on 26 September 2024, world leaders approved a political declaration committing to a clear set of targets and actions, including reducing the estimated 4.95 million human deaths associated with bacterial AMR annually by 10% by 2030.[7] The increasing interconnectedness of the world and the fact that new classes of antibiotics have not been developed and approved for more than 25 years highlight the extent to which antimicrobial resistance is a global health challenge.[130] A global action plan to tackle the growing problem of resistance to antibiotics and other antimicrobial medicines was endorsed at the Sixty-eighth World Health Assembly in May 2015.[89] One of the key objectives of the plan is to improve awareness and understanding of antimicrobial resistance through effective communication, education and training. This global action plan developed by the World Health Organization was created to combat the issue of antimicrobial resistance and was guided by the advice of countries and key stakeholders. The WHO's global action plan is composed of five key objectives that can be targeted through different means, and represents countries coming together to solve a major problem that can have future health consequences.[29] These objectives are as follows:
- improve awareness and understanding of antimicrobial resistance through effective communication, education and training.
- strengthen the knowledge and evidence base through surveillance and research.
- reduce the incidence of infection through effective sanitation, hygiene and infection prevention measures.
- optimize the use of antimicrobial medicines in human and animal health.
- develop the economic case for sustainable investment that takes account of the needs of all countries and to increase investment in new medicines, diagnostic tools, vaccines and other interventions.
Steps towards progress
- React based in Sweden has produced informative material on AMR for the general public.[131]
- Videos are being produced for the general public to generate interest and awareness.[132][133]
- The Irish Department of Health published a National Action Plan on Antimicrobial Resistance in October 2017.[134] The Strategy for the Control of Antimicrobial Resistance in Ireland (SARI), Iaunched in 2001 developed Guidelines for Antimicrobial Stewardship in Hospitals in Ireland[135] in conjunction with the Health Protection Surveillance Centre, these were published in 2009. Following their publication a public information campaign 'Action on Antibiotics[136]' was launched to highlight the need for a change in antibiotic prescribing. Despite this, antibiotic prescribing remains high with variance in adherence to guidelines.[137]
- The United Kingdom published a 20-year vision for antimicrobial resistance that sets out the goal of containing and controlling AMR by 2040.[138] The vision is supplemented by a 5-year action plan running from 2019 to 2024, building on the previous action plan (2013–2018).[139]
- The World Health Organization has published the 2024 Bacterial Priority Pathogens List which covers 15 families of antibiotic-resistant bacterial pathogens. Notable among these are gram-negative bacteria resistant to last-resort antibiotics, drug-resistant mycobacterium tuberculosis, and other high-burden resistant pathogens such as Salmonella, Shigella, Neisseria gonorrhoeae, Pseudomonas aeruginosa, and Staphylococcus aureus. The inclusion of these pathogens in the list underscores their global impact in terms of burden, as well as issues related to transmissibility, treatability, and prevention options. It also reflects the R&D pipeline of new treatments and emerging resistance trends.[140]
Antibiotic Awareness Week
[edit]The World Health Organization has promoted the first World Antibiotic Awareness Week running from 16 to 22 November 2015. The aim of the week is to increase global awareness of antibiotic resistance. It also wants to promote the correct usage of antibiotics across all fields in order to prevent further instances of antibiotic resistance.[141]
World Antibiotic Awareness Week has been held every November since 2015. For 2017, the Food and Agriculture Organization of the United Nations (FAO), the World Health Organization (WHO) and the World Organisation for Animal Health (OIE) are together calling for responsible use of antibiotics in humans and animals to reduce the emergence of antibiotic resistance.[142]
United Nations
In 2016 the Secretary-General of the United Nations convened the Interagency Coordination Group (IACG) on Antimicrobial Resistance.[143] The IACG worked with international organizations and experts in human, animal, and plant health to create a plan to fight antimicrobial resistance.[143] Their report released in April 2019 highlights the seriousness of antimicrobial resistance and the threat it poses to world health. It suggests five recommendations for member states to follow in order to tackle this increasing threat. The IACG recommendations are as follows:[144]
- Accelerate progress in countries
- Innovate to secure the future
- Collaborate for more effective action
- Invest for a sustainable response
- Strengthen accountability and global governance
Mechanisms and organisms
[edit]Bacteria
[edit]
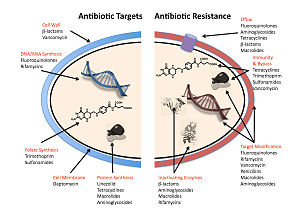
The five main mechanisms by which bacteria exhibit resistance to antibiotics are:
- Drug inactivation or modification: for example, enzymatic deactivation of penicillin G in some penicillin-resistant bacteria through the production of β-lactamases. Drugs may also be chemically modified through the addition of functional groups by transferase enzymes; for example, acetylation, phosphorylation, or adenylation are common resistance mechanisms to aminoglycosides. Acetylation is the most widely used mechanism and can affect a number of drug classes.[145][146]: 6–8
- Alteration of target- or binding site: for example, alteration of PBP—the binding target site of penicillins—in MRSA and other penicillin-resistant bacteria. Another protective mechanism found among bacterial species is ribosomal protection proteins. These proteins protect the bacterial cell from antibiotics that target the cell's ribosomes to inhibit protein synthesis. The mechanism involves the binding of the ribosomal protection proteins to the ribosomes of the bacterial cell, which in turn changes its conformational shape. This allows the ribosomes to continue synthesizing proteins essential to the cell while preventing antibiotics from binding to the ribosome to inhibit protein synthesis.[147]
- Alteration of metabolic pathway: for example, some sulfonamide-resistant bacteria do not require para-aminobenzoic acid (PABA), an important precursor for the synthesis of folic acid and nucleic acids in bacteria inhibited by sulfonamides, instead, like mammalian cells, they turn to using preformed folic acid.[148]
- Reduced drug accumulation: by decreasing drug permeability or increasing active efflux (pumping out) of the drugs across the cell surface[149] These pumps within the cellular membrane of certain bacterial species are used to pump antibiotics out of the cell before they are able to do any damage. They are often activated by a specific substrate associated with an antibiotic,[150] as in fluoroquinolone resistance.[151]
- Ribosome splitting and recycling: for example, drug-mediated stalling of the ribosome by lincomycin and erythromycin unstalled by a heat shock protein found in Listeria monocytogenes, which is a homologue of HflX from other bacteria. Liberation of the ribosome from the drug allows further translation and consequent resistance to the drug.[152]
There are several different types of germs that have developed a resistance over time.
The six pathogens causing most deaths associated with resistance are Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa. They were responsible for 929,000 deaths attributable to resistance and 3.57 million deaths associated with resistance in 2019.[17]
Penicillinase-producing Neisseria gonorrhoeae developed a resistance to penicillin in 1976. Another example is Azithromycin-resistant Neisseria gonorrhoeae, which developed a resistance to azithromycin in 2011.[153]
In gram-negative bacteria, plasmid-mediated resistance genes produce proteins that can bind to DNA gyrase, protecting it from the action of quinolones. Finally, mutations at key sites in DNA gyrase or topoisomerase IV can decrease their binding affinity to quinolones, decreasing the drug's effectiveness.[154]
Some bacteria are naturally resistant to certain antibiotics; for example, gram-negative bacteria are resistant to most β-lactam antibiotics due to the presence of β-lactamase. Antibiotic resistance can also be acquired as a result of either genetic mutation or horizontal gene transfer.[155] Although mutations are rare, with spontaneous mutations in the pathogen genome occurring at a rate of about 1 in 105 to 1 in 108 per chromosomal replication,[156] the fact that bacteria reproduce at a high rate allows for the effect to be significant. Given that lifespans and production of new generations can be on a timescale of mere hours, a new (de novo) mutation in a parent cell can quickly become an inherited mutation of widespread prevalence, resulting in the microevolution of a fully resistant colony. However, chromosomal mutations also confer a cost of fitness. For example, a ribosomal mutation may protect a bacterial cell by changing the binding site of an antibiotic but may result in slower growth rate.[157] Moreover, some adaptive mutations can propagate not only through inheritance but also through horizontal gene transfer. The most common mechanism of horizontal gene transfer is the transferring of plasmids carrying antibiotic resistance genes between bacteria of the same or different species via conjugation. However, bacteria can also acquire resistance through transformation, as in Streptococcus pneumoniae uptaking of naked fragments of extracellular DNA that contain antibiotic resistance genes to streptomycin,[158] through transduction, as in the bacteriophage-mediated transfer of tetracycline resistance genes between strains of S. pyogenes,[159] or through gene transfer agents, which are particles produced by the host cell that resemble bacteriophage structures and are capable of transferring DNA.[160]
Antibiotic resistance can be introduced artificially into a microorganism through laboratory protocols, sometimes used as a selectable marker to examine the mechanisms of gene transfer or to identify individuals that absorbed a piece of DNA that included the resistance gene and another gene of interest.[161]
Recent findings show no necessity of large populations of bacteria for the appearance of antibiotic resistance. Small populations of Escherichia coli in an antibiotic gradient can become resistant. Any heterogeneous environment with respect to nutrient and antibiotic gradients may facilitate antibiotic resistance in small bacterial populations. Researchers hypothesize that the mechanism of resistance evolution is based on four SNP mutations in the genome of E. coli produced by the gradient of antibiotic.[162]
In one study, which has implications for space microbiology, a non-pathogenic strain E. coli MG1655 was exposed to trace levels of the broad spectrum antibiotic chloramphenicol, under simulated microgravity (LSMMG, or Low Shear Modeled Microgravity) over 1000 generations. The adapted strain acquired resistance to not only chloramphenicol, but also cross-resistance to other antibiotics;[163] this was in contrast to the observation on the same strain, which was adapted to over 1000 generations under LSMMG, but without any antibiotic exposure; the strain in this case did not acquire any such resistance.[164] Thus, irrespective of where they are used, the use of an antibiotic would likely result in persistent resistance to that antibiotic, as well as cross-resistance to other antimicrobials.
In recent years, the emergence and spread of β-lactamases called carbapenemases has become a major health crisis.[165][166] One such carbapenemase is New Delhi metallo-beta-lactamase 1 (NDM-1),[167] an enzyme that makes bacteria resistant to a broad range of beta-lactam antibiotics. The most common bacteria that make this enzyme are gram-negative such as E. coli and Klebsiella pneumoniae, but the gene for NDM-1 can spread from one strain of bacteria to another by horizontal gene transfer.[168]
Viruses
[edit]Specific antiviral drugs are used to treat some viral infections. These drugs prevent viruses from reproducing by inhibiting essential stages of the virus's replication cycle in infected cells. Antivirals are used to treat HIV, hepatitis B, hepatitis C, influenza, herpes viruses including varicella zoster virus, cytomegalovirus and Epstein–Barr virus. With each virus, some strains have become resistant to the administered drugs.[169]
Antiviral drugs typically target key components of viral reproduction; for example, oseltamivir targets influenza neuraminidase, while guanosine analogs inhibit viral DNA polymerase. Resistance to antivirals is thus acquired through mutations in the genes that encode the protein targets of the drugs.
Resistance to HIV antivirals is problematic, and even multi-drug resistant strains have evolved.[170] One source of resistance is that many current HIV drugs, including NRTIs and NNRTIs, target reverse transcriptase; however, HIV-1 reverse transcriptase is highly error prone and thus mutations conferring resistance arise rapidly.[171] Resistant strains of the HIV virus emerge rapidly if only one antiviral drug is used.[172] Using three or more drugs together, termed combination therapy, has helped to control this problem, but new drugs are needed because of the continuing emergence of drug-resistant HIV strains.[173]
Fungi
[edit]Infections by fungi are a cause of high morbidity and mortality in immunocompromised persons, such as those with HIV/AIDS, tuberculosis or receiving chemotherapy.[174] The fungi Candida, Cryptococcus neoformans and Aspergillus fumigatus cause most of these infections and antifungal resistance occurs in all of them.[175] Multidrug resistance in fungi is increasing because of the widespread use of antifungal drugs to treat infections in immunocompromised individuals and the use of some agricultural antifungals.[92][176] Antifungal resistant disease is associated with increased mortality.
Some fungi (e.g. Candida krusei and fluconazole) exhibit intrinsic resistance to certain antifungal drugs or classes, whereas some species develop antifungal resistance to external pressures. Antifungal resistance is a One Health concern, driven by multiple extrinsic factors, including extensive fungicidal use, overuse of clinical antifungals, environmental change and host factors.[92]
In the USA fluconazole-resistant Candida species and azole resistance in Aspergillus fumigatus have been highlighted as a growing threat.[85]
More than 20 species of Candida can cause candidiasis infection, the most common of which is Candida albicans. Candida yeasts normally inhabit the skin and mucous membranes without causing infection. However, overgrowth of Candida can lead to candidiasis. Some Candida species (e.g. Candida glabrata) are becoming resistant to first-line and second-line antifungal agents such as echinocandins and azoles.[85]
The emergence of Candida auris as a potential human pathogen that sometimes exhibits multi-class antifungal drug resistance is concerning and has been associated with several outbreaks globally. The WHO has released a priority fungal pathogen list, including pathogens with antifungal resistance.[177]
The identification of antifungal resistance is undermined by limited classical diagnosis of infection, where a culture is lacking, preventing susceptibility testing.[92] National and international surveillance schemes for fungal disease and antifungal resistance are limited, hampering the understanding of the disease burden and associated resistance.[92] The application of molecular testing to identify genetic markers associating with resistance may improve the identification of antifungal resistance, but the diversity of mutations associated with resistance is increasing across the fungal species causing infection. In addition, a number of resistance mechanisms depend on up-regulation of selected genes (for instance reflux pumps) rather than defined mutations that are amenable to molecular detection.
Due to the limited number of antifungals in clinical use and the increasing global incidence of antifungal resistance, using the existing antifungals in combination might be beneficial in some cases but further research is needed. Similarly, other approaches that might help to combat the emergence of antifungal resistance could rely on the development of host-directed therapies such as immunotherapy or vaccines.[92]
Parasites
[edit]The protozoan parasites that cause the diseases malaria, trypanosomiasis, toxoplasmosis, cryptosporidiosis and leishmaniasis are important human pathogens.[178]
Malarial parasites that are resistant to the drugs that are currently available to infections are common and this has led to increased efforts to develop new drugs.[179] Resistance to recently developed drugs such as artemisinin has also been reported. The problem of drug resistance in malaria has driven efforts to develop vaccines.[180]
Trypanosomes are parasitic protozoa that cause African trypanosomiasis and Chagas disease (American trypanosomiasis).[181][182] There are no vaccines to prevent these infections so drugs such as pentamidine and suramin, benznidazole and nifurtimox are used to treat infections. These drugs are effective but infections caused by resistant parasites have been reported.[178]
Leishmaniasis is caused by protozoa and is an important public health problem worldwide, especially in sub-tropical and tropical countries. Drug resistance has "become a major concern".[183]
Global and genomic data
[edit]

In 2022, genomic epidemiologists reported results from a global survey of antimicrobial resistance via genomic wastewater-based epidemiology, finding large regional variations, providing maps, and suggesting resistance genes are also passed on between microbial species that are not closely related.[185][184] The WHO provides the Global Antimicrobial Resistance and Use Surveillance System (GLASS) reports which summarize annual (e.g. 2020's) data on international AMR, also including an interactive dashboard.[186][187]
Epidemiology
[edit]United Kingdom
[edit]Public Health England reported that the total number of antibiotic resistant infections in England rose by 9% from 55,812 in 2017 to 60,788 in 2018, but antibiotic consumption had fallen by 9% from 20.0 to 18.2 defined daily doses per 1,000 inhabitants per day between 2014 and 2018.[188]
United States
[edit]The Centers for Disease Control and Prevention reported that more than 2.8 million cases of antibiotic resistance have been reported. However, in 2019 overall deaths from antibiotic-resistant infections decreased by 18% and deaths in hospitals decreased by 30%.[189]
The COVID pandemic caused a reversal of much of the progress made on attenuating the effects of antibiotic resistance, resulting in more antibiotic use, more resistant infections, and less data on preventive action.[190] Hospital-onset infections and deaths both increased by 15% in 2020, and significantly higher rates of infections were reported for 4 out of 6 types of healthcare associated infections.[191]
History
[edit]The 1950s to 1970s represented the golden age of antibiotic discovery, where countless new classes of antibiotics were discovered to treat previously incurable diseases such as tuberculosis and syphilis.[192] However, since that time the discovery of new classes of antibiotics has been almost nonexistent, and represents a situation that is especially problematic considering the resiliency of bacteria[193] shown over time and the continued misuse and overuse of antibiotics in treatment.[194]
The phenomenon of antimicrobial resistance caused by overuse of antibiotics was predicted as early as 1945 by Alexander Fleming who said "The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily under-dose himself and by exposing his microbes to nonlethal quantities of the drug make them resistant."[195][196] Without the creation of new and stronger antibiotics an era where common infections and minor injuries can kill, and where complex procedures such as surgery and chemotherapy become too risky, is a very real possibility.[197] Antimicrobial resistance can lead to epidemics of enormous proportions if preventive actions are not taken. In this day and age current antimicrobial resistance leads to longer hospital stays, higher medical costs, and increased mortality.[194]
Society and culture
[edit]Innovation policy
[edit]Since the mid-1980s pharmaceutical companies have invested in medications for cancer or chronic disease that have greater potential to make money and have "de-emphasized or dropped development of antibiotics".[198] On 20 January 2016 at the World Economic Forum in Davos, Switzerland, more than "80 pharmaceutical and diagnostic companies" from around the world called for "transformational commercial models" at a global level to spur research and development on antibiotics and on the "enhanced use of diagnostic tests that can rapidly identify the infecting organism".[198] A number of countries are considering or implementing delinked payment models for new antimicrobials whereby payment is based on value rather than volume of drug sales. This offers the opportunity to pay for valuable new drugs even if they are reserved for use in relatively rare drug resistant infections.[199]
Legal frameworks
[edit]Some global health scholars have argued that a global, legal framework is needed to prevent and control antimicrobial resistance.[200][201][202][203] For instance, binding global policies could be used to create antimicrobial use standards, regulate antibiotic marketing, and strengthen global surveillance systems.[202][200] Ensuring compliance of involved parties is a challenge.[202] Global antimicrobial resistance policies could take lessons from the environmental sector by adopting strategies that have made international environmental agreements successful in the past such as: sanctions for non-compliance, assistance for implementation, majority vote decision-making rules, an independent scientific panel, and specific commitments.[204]
United States
[edit]This section needs to be updated. (October 2023) |
For the United States 2016 budget, U.S. president Barack Obama proposed to nearly double the amount of federal funding to "combat and prevent" antibiotic resistance to more than $1.2 billion.[205] Many international funding agencies like USAID, DFID, SIDA and Bill & Melinda Gates Foundation have pledged money for developing strategies to counter antimicrobial resistance.[citation needed]
On 27 March 2015, the White House released a comprehensive plan to address the increasing need for agencies to combat the rise of antibiotic-resistant bacteria. The Task Force for Combating Antibiotic-Resistant Bacteria developed The National Action Plan for Combating Antibiotic-Resistant Bacteria with the intent of providing a roadmap to guide the US in the antibiotic resistance challenge and with hopes of saving many lives. This plan outlines steps taken by the Federal government over the next five years needed in order to prevent and contain outbreaks of antibiotic-resistant infections; maintain the efficacy of antibiotics already on the market; and to help to develop future diagnostics, antibiotics, and vaccines.[206]
The Action Plan was developed around five goals with focuses on strengthening health care, public health veterinary medicine, agriculture, food safety and research, and manufacturing. These goals, as listed by the White House, are as follows:
- Slow the Emergence of Resistant Bacteria and Prevent the Spread of Resistant Infections
- Strengthen National One-Health Surveillance Efforts to Combat Resistance
- Advance Development and use of Rapid and Innovative Diagnostic Tests for Identification and Characterization of Resistant Bacteria
- Accelerate Basic and Applied Research and Development for New Antibiotics, Other Therapeutics, and Vaccines
- Improve International Collaboration and Capacities for Antibiotic Resistance Prevention, Surveillance, Control and Antibiotic Research and Development
The following are goals set to meet by 2020:[206]
- Establishment of antimicrobial programs within acute care hospital settings
- Reduction of inappropriate antibiotic prescription and use by at least 50% in outpatient settings and 20% inpatient settings
- Establishment of State Antibiotic Resistance (AR) Prevention Programs in all 50 states
- Elimination of the use of medically important antibiotics for growth promotion in food-producing animals.
Current Status of AMR in the U.S.
As of 2023, antimicrobial resistance (AMR) remains a significant public health threat in the United States. According to the Centers for Disease Control and Prevention's 2023 Report on Antibiotic Resistance Threats, over 2.8 million antibiotic-resistant infections occur in the U.S. each year, leading to at least 35,000 deaths annually.[207] Among the most concerning resistant pathogens are Carbapenem-resistant Enterobacteriaceae (CRE), Methicillin-resistant Staphylococcus aureus (MRSA), and Clostridioides difficile (C. diff), all of which continue to be responsible for severe healthcare-associated infections (HAIs).
The COVID-19 pandemic led to a significant disruption in healthcare, with an increase in the use of antibiotics during the treatment of viral infections. This rise in antibiotic prescribing, coupled with overwhelmed healthcare systems, contributed to a resurgence in AMR during the pandemic years. A 2021 CDC report identified a sharp increase in HAIs caused by resistant pathogens in COVID-19 patients, a trend that has persisted into 2023.[208] Recent data suggest that although antibiotic use has decreased since the pandemic, some resistant pathogens remain prevalent in healthcare settings.[207]
The CDC has also expanded its Get Ahead of Sepsis campaign in 2023, focusing on raising awareness of AMR's role in sepsis and promoting the judicious use of antibiotics in both healthcare and community settings.[209] This initiative has reached millions through social media, healthcare facilities, and public health outreach, aiming to educate the public on the importance of preventing infections and reducing antibiotic misuse.
Policies
[edit]According to World Health Organization, policymakers can help tackle resistance by strengthening resistance-tracking and laboratory capacity and by regulating and promoting the appropriate use of medicines.[16] Policymakers and industry can help tackle resistance by: fostering innovation and research and development of new tools; and promoting cooperation and information sharing among all stakeholders.[16]
The U.S. government continues to prioritize AMR mitigation through policy and legislation. In 2023, the National Action Plan for Combating Antibiotic-Resistant Bacteria (CARB) 2023-2028 was released, outlining strategic objectives for reducing antibiotic-resistant infections, advancing infection prevention, and accelerating research on new antibiotics.[210] The plan also emphasizes the importance of improving antibiotic stewardship across healthcare, agriculture, and veterinary settings. Furthermore, the PASTEUR Act (Pioneering Antimicrobial Subscriptions to End Upsurging Resistance) has gained momentum in Congress. If passed, the bill would create a subscription-based payment model to incentivize the development of new antimicrobial drugs, while supporting antimicrobial stewardship programs to reduce the misuse of existing antibiotics.[211] This legislation is considered a critical step toward addressing the economic barriers to developing new antimicrobials.
Policy evaluation
[edit]Measuring the costs and benefits of strategies to combat AMR is difficult and policies may only have effects in the distant future. In other infectious diseases this problem has been addressed by using mathematical models. More research is needed to understand how AMR develops and spreads so that mathematical modelling can be used to anticipate the likely effects of different policies.[212]
Further research
[edit]Rapid testing and diagnostics
[edit]
Distinguishing infections requiring antibiotics from self-limiting ones is clinically challenging. In order to guide appropriate use of antibiotics and prevent the evolution and spread of antimicrobial resistance, diagnostic tests that provide clinicians with timely, actionable results are needed.
Acute febrile illness is a common reason for seeking medical care worldwide and a major cause of morbidity and mortality. In areas with decreasing malaria incidence, many febrile patients are inappropriately treated for malaria, and in the absence of a simple diagnostic test to identify alternative causes of fever, clinicians presume that a non-malarial febrile illness is most likely a bacterial infection, leading to inappropriate use of antibiotics. Multiple studies have shown that the use of malaria rapid diagnostic tests without reliable tools to distinguish other fever causes has resulted in increased antibiotic use.[213]
Antimicrobial susceptibility testing (AST) can facilitate a precision medicine approach to treatment by helping clinicians to prescribe more effective and targeted antimicrobial therapy.[214] At the same time with traditional phenotypic AST it can take 12 to 48 hours to obtain a result due to the time taken for organisms to grow on/in culture media.[215] Rapid testing, possible from molecular diagnostics innovations, is defined as "being feasible within an 8-h working shift".[215] There are several commercial Food and Drug Administration-approved assays available which can detect AMR genes from a variety of specimen types. Progress has been slow due to a range of reasons including cost and regulation.[216] Genotypic AMR characterisation methods are, however, being increasingly used in combination with machine learning algorithms in research to help better predict phenotypic AMR from organism genotype.[217][218]
Optical techniques such as phase contrast microscopy in combination with single-cell analysis are another powerful method to monitor bacterial growth. In 2017, scientists from Uppsala University in Sweden published a method[219] that applies principles of microfluidics and cell tracking, to monitor bacterial response to antibiotics in less than 30 minutes overall manipulation time. This invention was awarded the 8M£ Longitude Prize on AMR in 2024. Recently, this platform has been advanced by coupling microfluidic chip with optical tweezing[220] in order to isolate bacteria with altered phenotype directly from the analytical matrix.
Rapid diagnostic methods have also been trialled as antimicrobial stewardship interventions to influence the healthcare drivers of AMR. Serum procalcitonin measurement has been shown to reduce mortality rate, antimicrobial consumption and antimicrobial-related side-effects in patients with respiratory infections, but impact on AMR has not yet been demonstrated.[221] Similarly, point of care serum testing of the inflammatory biomarker C-reactive protein has been shown to influence antimicrobial prescribing rates in this patient cohort, but further research is required to demonstrate an effect on rates of AMR.[222] Clinical investigation to rule out bacterial infections are often done for patients with pediatric acute respiratory infections. Currently it is unclear if rapid viral testing affects antibiotic use in children.[223]
Vaccines
[edit]Vaccines are an essential part of the response to reduce AMR as they prevent infections, reduce the use and overuse of antimicrobials, and slow the emergence and spread of drug-resistant pathogens.[7] Microorganisms usually do not develop resistance to vaccines because vaccines reduce the spread of the infection and target the pathogen in multiple ways in the same host and possibly in different ways between different hosts. Furthermore, if the use of vaccines increases, there is evidence that antibiotic resistant strains of pathogens will decrease; the need for antibiotics will naturally decrease as vaccines prevent infection before it occurs.[224] A 2024 report by WHO finds that vaccines against 24 pathogens could reduce the number of antibiotics needed by 22% or 2.5 billion defined daily doses globally every year.[7] If vaccines could be rolled out against all the evaluated pathogens, they could save a third of the hospital costs associated with AMR.[7] Vaccinated people have fewer infections and are protected against potential complications from secondary infections that may need antimicrobial medicines or require admission to hospital.[7] However, there are well documented cases of vaccine resistance, although these are usually much less of a problem than antimicrobial resistance.[225][226]
While theoretically promising, antistaphylococcal vaccines have shown limited efficacy, because of immunological variation between Staphylococcus species, and the limited duration of effectiveness of the antibodies produced. Development and testing of more effective vaccines is underway.[227]
Two registrational trials have evaluated vaccine candidates in active immunization strategies against S. aureus infection. In a phase II trial, a bivalent vaccine of capsular proteins 5 & 8 was tested in 1804 hemodialysis patients with a primary fistula or synthetic graft vascular access. After 40 weeks following vaccination a protective effect was seen against S. aureus bacteremia, but not at 54 weeks following vaccination.[228] Based on these results, a second trial was conducted which failed to show efficacy.[229]
Merck tested V710, a vaccine targeting IsdB, in a blinded randomized trial in patients undergoing median sternotomy. The trial was terminated after a higher rate of multiorgan system failure–related deaths was found in the V710 recipients. Vaccine recipients who developed S. aureus infection were five times more likely to die than control recipients who developed S. aureus infection.[230]
Numerous investigators have suggested that a multiple-antigen vaccine would be more effective, but a lack of biomarkers defining human protective immunity keep these proposals in the logical, but strictly hypothetical arena.[229]
Antibody therapy
[edit]Antibodies are promising against antimicrobial resistance. Monoclonal antibodies (mAbs) target bacterial virulence factors, aiding in bacterial destruction through various mechanisms. Three FDA-approved antibodies target B. anthracis and C. difficile toxins.[231][232] Innovative strategies include DSTA4637S, an antibody-antibiotic conjugate, and MEDI13902, a bispecific antibody targeting Pseudomonas aeruginosa components.[232]
Alternating therapy
[edit]Alternating therapy is a proposed method in which two or three antibiotics are taken in a rotation versus taking just one antibiotic such that bacteria resistant to one antibiotic are killed when the next antibiotic is taken. Studies have found that this method reduces the rate at which antibiotic resistant bacteria emerge in vitro relative to a single drug for the entire duration.[233]
Studies have found that bacteria that evolve antibiotic resistance towards one group of antibiotic may become more sensitive to others.[234] This phenomenon can be used to select against resistant bacteria using an approach termed collateral sensitivity cycling, which has recently been found to be relevant in developing treatment strategies for chronic infections caused by Pseudomonas aeruginosa.[235] Despite its promise, large-scale clinical and experimental studies revealed limited evidence of susceptibility to antibiotic cycling across various pathogens.[236][237]
Development of new drugs
[edit]Since the discovery of antibiotics, research and development (R&D) efforts have provided new drugs in time to treat bacteria that became resistant to older antibiotics, but in the 2000s there has been concern that development has slowed enough that seriously ill people may run out of treatment options.[238][239] Another concern is that practitioners may become reluctant to perform routine surgeries because of the increased risk of harmful infection.[240] Backup treatments can have serious side-effects; for example, antibiotics like aminoglycosides (such as amikacin, gentamicin, kanamycin, streptomycin, etc.) used for the treatment of drug-resistant tuberculosis and cystic fibrosis can cause respiratory disorders, deafness and kidney failure.[241][242]
The potential crisis at hand is the result of a marked decrease in industry research and development.[243][244] Poor financial investment in antibiotic research has exacerbated the situation.[245][243] The pharmaceutical industry has little incentive to invest in antibiotics because of the high risk and because the potential financial returns are less likely to cover the cost of development than for other pharmaceuticals.[246] In 2011, Pfizer, one of the last major pharmaceutical companies developing new antibiotics, shut down its primary research effort, citing poor shareholder returns relative to drugs for chronic illnesses.[247] However, small and medium-sized pharmaceutical companies are still active in antibiotic drug research. In particular, apart from classical synthetic chemistry methodologies, researchers have developed a combinatorial synthetic biology platform on single cell level in a high-throughput screening manner to diversify novel lanthipeptides.[248]
In the 5–10 years since 2010, there has been a significant change in the ways new antimicrobial agents are discovered and developed – principally via the formation of public-private funding initiatives. These include CARB-X,[249] which focuses on nonclinical and early phase development of novel antibiotics, vaccines, rapid diagnostics; Novel Gram Negative Antibiotic (GNA-NOW),[250] which is part of the EU's Innovative Medicines Initiative; and Replenishing and Enabling the Pipeline for Anti-infective Resistance Impact Fund (REPAIR).[251] Later stage clinical development is supported by the AMR Action Fund, which in turn is supported by multiple investors with the aim of developing 2–4 new antimicrobial agents by 2030. The delivery of these trials is facilitated by national and international networks supported by the Clinical Research Network of the National Institute for Health and Care Research (NIHR), European Clinical Research Alliance in Infectious Diseases (ECRAID) and the recently formed ADVANCE-ID, which is a clinical research network based in Asia.[252] The Global Antimicrobial Research and Development Partnership (GARDP) is generating new evidence for global AMR threats such as neonatal sepsis, treatment of serious bacterial infections and sexually transmitted infections as well as addressing global access to new and strategically important antibacterial drugs.[253]
The discovery and development of new antimicrobial agents has been facilitated by regulatory advances, which have been principally led by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA). These processes are increasingly aligned although important differences remain and drug developers must prepare separate documents. New development pathways have been developed to help with the approval of new antimicrobial agents that address unmet needs such as the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD). These new pathways are required because of difficulties in conducting large definitive phase III clinical trials in a timely way.
Some of the economic impediments to the development of new antimicrobial agents have been addressed by innovative reimbursement schemes that delink payment of antimicrobials from volume-based sales. In the UK, a market entry reward scheme has been pioneered by the National Institute for Clinical Excellence (NICE) whereby an annual subscription fee is paid for use of strategically valuable antimicrobial agents – cefiderocol and ceftazidime-aviabactam are the first agents to be used in this manner and the scheme is potential blueprint for comparable programs in other countries.
The available classes of antifungal drugs are still limited but as of 2021 novel classes of antifungals are being developed and are undergoing various stages of clinical trials to assess performance.[254]
Scientists have started using advanced computational approaches with supercomputers for the development of new antibiotic derivatives to deal with antimicrobial resistance.[244][255][256]
Biomaterials
[edit]Using antibiotic-free alternatives in bone infection treatment may help decrease the use of antibiotics and thus antimicrobial resistance.[36] The bone regeneration material bioactive glass S53P4 has shown to effectively inhibit the bacterial growth of up to 50 clinically relevant bacteria including MRSA and MRSE.[257][258][259]
Nanomaterials
[edit]During the last decades, copper and silver nanomaterials have demonstrated appealing features for the development of a new family of antimicrobial agents.[260] Nanoparticles (1-100 nm) show unique properties and promise as antimicrobial agents against resistant bacteria. Silver (AgNPs) and gold nanoparticles (AuNPs) are extensively studied, disrupting bacterial cell membranes and interfering with protein synthesis. Zinc oxide (ZnO NPs), copper (CuNPs), and silica (SiNPs) nanoparticles also exhibit antimicrobial properties. However, high synthesis costs, potential toxicity, and instability pose challenges. To overcome these, biological synthesis methods and combination therapies with other antimicrobials are explored. Enhanced biocompatibility and targeting are also under investigation to improve efficacy.[232]
Rediscovery of ancient treatments
[edit]Similar to the situation in malaria therapy, where successful treatments based on ancient recipes have been found,[261] there has already been some success in finding and testing ancient drugs and other treatments that are effective against AMR bacteria.[262]
Computational community surveillance
[edit]One of the key tools identified by the WHO and others for the fight against rising antimicrobial resistance is improved surveillance of the spread and movement of AMR genes through different communities and regions. Recent advances in high-throughput DNA sequencing as a result of the Human Genome Project have resulted in the ability to determine the individual microbial genes in a sample.[263] Along with the availability of databases of known antimicrobial resistance genes, such as the Comprehensive Antimicrobial Resistance Database (CARD)[264][265] and ResFinder,[266][267] this allows the identification of all the antimicrobial resistance genes within the sample – the so-called "resistome". In doing so, a profile of these genes within a community or environment can be determined, providing insights into how antimicrobial resistance is spreading through a population and allowing for the identification of resistance that is of concern.[263]
Phage therapy
[edit]Phage therapy is the therapeutic use of bacteriophages to treat pathogenic bacterial infections.[268] Phage therapy has many potential applications in human medicine as well as dentistry, veterinary science, and agriculture.[269]
Phage therapy relies on the use of naturally occurring bacteriophages to infect and lyse bacteria at the site of infection in a host. Due to current advances in genetics and biotechnology these bacteriophages can possibly be manufactured to treat specific infections.[270] Phages can be bioengineered to target multidrug-resistant bacterial infections, and their use involves the added benefit of preventing the elimination of beneficial bacteria in the human body.[31] Phages destroy bacterial cell walls and membrane through the use of lytic proteins which kill bacteria by making many holes from the inside out.[271] Bacteriophages can even possess the ability to digest the biofilm that many bacteria develop that protect them from antibiotics in order to effectively infect and kill bacteria. Bioengineering can play a role in creating successful bacteriophages.[271]
Understanding the mutual interactions and evolutions of bacterial and phage populations in the environment of a human or animal body is essential for rational phage therapy.[272]
Bacteriophagics are used against antibiotic resistant bacteria in Georgia (George Eliava Institute) and in one institute in Wrocław, Poland.[273][274] Bacteriophage cocktails are common drugs sold over the counter in pharmacies in eastern countries.[275][276] In Belgium, four patients with severe musculoskeletal infections received bacteriophage therapy with concomitant antibiotics. After a single course of phage therapy, no recurrence of infection occurred and no severe side-effects related to the therapy were detected.[277]
See also
[edit]- Alliance for the Prudent Use of Antibiotics
- Antimicrobial resistance in Australia
- Broad-spectrum antibiotic
- Colonisation resistance
- Drug of last resort
- Genetic engineering
- (KPC) antibacterial resistance gene
- Multidrug-resistant Gram-negative bacteria
- Multidrug-resistant tuberculosis
- New Delhi metallo-beta-lactamase 1
- Persister cells
- Resistance-nodulation-cell division superfamily (RND)
- Resistome
References
[edit]- ^ Kirby-Bauer Disk Diffusion Susceptibility Test Protocol Archived 26 June 2011 at the Wayback Machine, Jan Hudzicki, ASM
- ^ a b "About Antimicrobial Resistance". US Centers for Disease Control and Prevention. 22 April 2024. Retrieved 11 October 2024.
- ^ a b c "Antimicrobial resistance Fact sheet N°194". who.int. April 2014. Archived from the original on 10 March 2015. Retrieved 7 March 2015.
- ^ a b Tanwar J, Das S, Fatima Z, Hameed S (2014). "Multidrug resistance: an emerging crisis". Interdisciplinary Perspectives on Infectious Diseases. 2014: 541340. doi:10.1155/2014/541340. PMC 4124702. PMID 25140175.
- ^ Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. (March 2012). "Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance". Clinical Microbiology and Infection. 18 (3): 268–281. doi:10.1111/j.1469-0691.2011.03570.x. PMID 21793988.
- ^ a b c d Dabour R, Meirson T, Samson AO (December 2016). "Global antibiotic resistance is mostly periodic". Journal of Global Antimicrobial Resistance. 7: 132–134. doi:10.1016/j.jgar.2016.09.003. PMID 27788414.
- ^ a b c d e f g h i j "Better use of vaccines could reduce antibiotic use by 2.5 billion doses annually, says WHO". World Health Organization. 10 October 2024. Retrieved 11 October 2024.
- ^ a b Saha M, Sarkar A (December 2021). "Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century". Journal of Xenobiotics. 11 (4): 197–214. doi:10.3390/jox11040013. PMC 8708150. PMID 34940513.
- ^ Swedish work on containment of antibiotic resistance – Tools, methods and experiences (PDF). Stockholm: Public Health Agency of Sweden. 2014. pp. 16–17, 121–128. ISBN 978-91-7603-011-0. Archived (PDF) from the original on 23 July 2015. Retrieved 23 July 2015.
- ^ "Antimicrobial resistance". www.who.int. Retrieved 20 November 2024.
- ^ Chanel S, Doherty B (10 September 2020). "'Superbugs' a far greater risk than Covid in Pacific, scientist warns". The Guardian. ISSN 0261-3077. Archived from the original on 5 December 2022. Retrieved 14 September 2020.
- ^ Rodríguez-Baño J, Rossolini GM, Schultsz C, Tacconelli E, Murthy S, Ohmagari N, et al. (March 2021). "Key considerations on the potential impacts of the COVID-19 pandemic on antimicrobial resistance research and surveillance". Trans R Soc Trop Med Hyg. 115 (10): 1122–1129. doi:10.1093/trstmh/trab048. PMC 8083707. PMID 33772597.
- ^ "What is Drug Resistance?". niaid.nih.gov. Archived from the original on 27 July 2015. Retrieved 26 July 2015.
- ^ "CDC: Get Smart: Know When Antibiotics Work". Cdc.gov. 29 May 2018. Archived from the original on 29 April 2015. Retrieved 12 June 2013.
- ^ MacGowan A, Macnaughton E (1 October 2017). "Antibiotic resistance". Medicine. 45 (10): 622–628. doi:10.1016/j.mpmed.2017.07.006.
- ^ a b c d e "WHO's first global report on antibiotic resistance reveals serious, worldwide threat to public health" Archived 2 May 2014 at the Wayback Machine Retrieved 2 May 2014
- ^ a b c Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. (Antimicrobial Resistance Collaborators) (February 2022). "Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis". Lancet. 399 (10325): 629–655. doi:10.1016/S0140-6736(21)02724-0. PMC 8841637. PMID 35065702. S2CID 246077406.
- ^ a b "Antibiotic resistance". who.int. Archived from the original on 21 May 2021. Retrieved 16 March 2020.
- ^ Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. (January 2019). "Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis". The Lancet. Infectious Diseases. 19 (1): 56–66. doi:10.1016/S1473-3099(18)30605-4. PMC 6300481. PMID 30409683.
- ^ "Antibiotic-resistant bacteria responsible for over 33,000 deaths in Europe in 2015, study finds". The Pharmaceutical Journal. 7 November 2018. Archived from the original on 28 March 2023. Retrieved 28 March 2023.
- ^ Mestrovic T, Robles Aguilar G, Swetschinski LR, Ikuta KS, Gray AP, Davis Weaver N, et al. (November 2022). "The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis". The Lancet. Public Health. 7 (11): e897–e913. doi:10.1016/S2468-2667(22)00225-0. hdl:10023/26218. PMC 9630253. PMID 36244350.
- ^ "Antimicrobial Resistance " Cambridge Medicine Journal". Retrieved 27 February 2020.[permanent dead link]
- ^ a b Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. (January 2016). "Understanding the mechanisms and drivers of antimicrobial resistance". Lancet. 387 (10014): 176–87. doi:10.1016/S0140-6736(15)00473-0. hdl:10044/1/32225. PMID 26603922. S2CID 1944665. Archived from the original on 14 April 2022. Retrieved 5 December 2021.
- ^ a b "CDC Newsroom". CDC. 1 January 2016. Archived from the original on 9 March 2023. Retrieved 28 February 2023.
- ^ a b Michael CA, Dominey-Howes D, Labbate M (2014). "The antimicrobial resistance crisis: causes, consequences, and management". Frontiers in Public Health. 2: 145. doi:10.3389/fpubh.2014.00145. PMC 4165128. PMID 25279369.
- ^ a b "Natural selection". evolution.berkeley.edu. Archived from the original on 30 October 2019. Retrieved 10 March 2020.
- ^ a b Larsen J, Raisen CL, Ba X, Sadgrove NJ, Padilla-González GF, Simmonds MS, et al. (February 2022). "Emergence of methicillin resistance predates the clinical use of antibiotics". Nature. 602 (7895): 135–141. Bibcode:2022Natur.602..135L. doi:10.1038/s41586-021-04265-w. PMC 8810379. PMID 34987223.
- ^ Crits-Christoph A, Hallowell HA, Koutouvalis K, Suez J (31 December 2022). "Good microbes, bad genes? The dissemination of antimicrobial resistance in the human microbiome". Gut Microbes. 14 (1): 2055944. doi:10.1080/19490976.2022.2055944. PMC 8959533. PMID 35332832.
- ^ a b c Ferri M, Ranucci E, Romagnoli P, Giaccone V (September 2017). "Antimicrobial resistance: A global emerging threat to public health systems". Critical Reviews in Food Science and Nutrition. 57 (13): 2857–2876. doi:10.1080/10408398.2015.1077192. PMID 26464037. S2CID 24549694.
- ^ "Global Database for Tracking Antimicrobial Resistance (AMR) Country Self- Assessment Survey (TrACSS)". amrcountryprogress.org. Archived from the original on 28 March 2023. Retrieved 28 March 2023.
- ^ a b c d Rather IA, Kim BC, Bajpai VK, Park YH (May 2017). "Self-medication and antibiotic resistance: Crisis, current challenges, and prevention". Saudi Journal of Biological Sciences. 24 (4): 808–812. doi:10.1016/j.sjbs.2017.01.004. PMC 5415144. PMID 28490950.
- ^ a b Torres NF, Chibi B, Middleton LE, Solomon VP, Mashamba-Thompson TP (March 2019). "Evidence of factors influencing self-medication with antibiotics in low and middle-income countries: a systematic scoping review". Public Health. 168: 92–101. doi:10.1016/j.puhe.2018.11.018. PMID 30716570. S2CID 73434085.
- ^ Ayukekbong JA, Ntemgwa M, Atabe AN (15 May 2017). "The threat of antimicrobial resistance in developing countries: causes and control strategies". Antimicrobial Resistance and Infection Control. 6 (1): 47. doi:10.1186/s13756-017-0208-x. PMC 5433038. PMID 28515903.
- ^ Chen C, Patterson B, Simpson R, Li Y, Chen Z, Lv Q, et al. (9 August 2022). "Do fluoroquinolones increase aortic aneurysm or dissection incidence and mortality? A systematic review and meta-analysis". Frontiers in Cardiovascular Medicine. 9: 949538. doi:10.3389/fcvm.2022.949538. PMC 9396038. PMID 36017083.
- ^ Shu Y, Zhang Q, He X, Liu Y, Wu P, Chen L (6 September 2022). "Fluoroquinolone-associated suspected tendonitis and tendon rupture: A pharmacovigilance analysis from 2016 to 2021 based on the FAERS database". Frontiers in Pharmacology. 13: 990241. doi:10.3389/fphar.2022.990241. PMC 9486157. PMID 36147351.
- ^ a b c Ventola CL (April 2015). "The antibiotic resistance crisis: part 1: causes and threats". P & T. 40 (4): 277–83. PMC 4378521. PMID 25859123.
- ^ Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM, et al. (May 2016). "Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011". JAMA. 315 (17): 1864–73. doi:10.1001/jama.2016.4151. PMID 27139059.
- ^ Strachan CR, Davies J (February 2017). "The Whys and Wherefores of Antibiotic Resistance". Cold Spring Harbor Perspectives in Medicine. 7 (2): a025171. doi:10.1101/cshperspect.a025171. PMC 5287056. PMID 27793964.
- ^ Harris A, Chandramohan S, Awali RA, Grewal M, Tillotson G, Chopra T (August 2019). "Physicians' attitude and knowledge regarding antibiotic use and resistance in ambulatory settings". American Journal of Infection Control. 47 (8): 864–868. doi:10.1016/j.ajic.2019.02.009. PMID 30926215. S2CID 88482220.
- ^ Joshi MP (17 February 2021). "Don't let Covid boost another killer". Knowable Magazine. doi:10.1146/knowable-021621-1. Archived from the original on 22 October 2021. Retrieved 10 August 2022.
- ^ Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. (December 2020). "Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing". Clinical Infectious Diseases. 71 (9): 2459–2468. doi:10.1093/cid/ciaa530. PMC 7197596. PMID 32358954.
- ^ Barnes S. "Rutgers study finds antibiotic overuse is caused by misconceptions, financial incentives". The Daily Targum. Archived from the original on 6 December 2021. Retrieved 16 February 2021.
- ^ Blaser MJ, Melby MK, Lock M, Nichter M (February 2021). "Accounting for variation in and overuse of antibiotics among humans". BioEssays. 43 (2): e2000163. doi:10.1002/bies.202000163. PMID 33410142. S2CID 230811912.
- ^ "Antimicrobials | American Veterinary Medical Association". avma.org. Archived from the original on 24 April 2024. Retrieved 24 April 2024.
- ^ Caneschi A, Bardhi A, Barbarossa A, Zaghini A (March 2023). "The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review". Antibiotics. 12 (3): 487. doi:10.3390/antibiotics12030487. PMC 10044628. PMID 36978354.
- ^ "Has COVID-19 made the superbug crisis worse?". Global News. Archived from the original on 12 February 2022. Retrieved 12 February 2022.
- ^ Lucien MA, Canarie MF, Kilgore PE, Jean-Denis G, Fénélon N, Pierre M, et al. (March 2021). "Antibiotics and antimicrobial resistance in the COVID-19 era: Perspective from resource-limited settings". International Journal of Infectious Diseases. 104: 250–254. doi:10.1016/j.ijid.2020.12.087. PMC 7796801. PMID 33434666.
- ^ "COVID-19 & Antibiotic Resistance". Centers for Disease Control and Prevention. 18 November 2021. Archived from the original on 21 February 2022. Retrieved 21 February 2022.
- ^ Knight GM, Glover RE, McQuaid CF, Olaru ID, Gallandat K, Leclerc QJ, et al. (February 2021). "Antimicrobial resistance and COVID-19: Intersections and implications". eLife. 10. doi:10.7554/eLife.64139. PMC 7886324. PMID 33588991. S2CID 231936902.
- ^ Lu J, Guo J (January 2021). "Disinfection spreads antimicrobial resistance". Science. 371 (6528): 474. Bibcode:2021Sci...371..474L. doi:10.1126/science.abg4380. PMID 33510019. S2CID 231730007.
- ^ Lobie TA, Roba AA, Booth JA, Kristiansen KI, Aseffa A, Skarstad K, et al. (October 2021). "Antimicrobial resistance: A challenge awaiting the post-COVID-19 era". International Journal of Infectious Diseases. 111: 322–325. doi:10.1016/j.ijid.2021.09.003. PMC 8425743. PMID 34508864. S2CID 237444117.
- ^ "World Leaders Approve Milestone Commitment To Reduce Deaths From Antibiotic Resistance By 10% By 2030 - Health Policy Watch". 26 September 2024. Retrieved 28 September 2024.
- ^ "UN General Assembly High-Level Meeting on antimicrobial resistance 2024". www.who.int. Retrieved 28 September 2024.
- ^ Musoke D, Namata C, Lubega GB, Niyongabo F, Gonza J, Chidziwisano K, et al. (October 2021). "The role of Environmental Health in preventing antimicrobial resistance in low- and middle-income countries". Environmental Health and Preventive Medicine. 26 (1): 100. Bibcode:2021EHPM...26..100M. doi:10.1186/s12199-021-01023-2. PMC 8493696. PMID 34610785.
- ^ a b c d e f Fletcher S (July 2015). "Understanding the contribution of environmental factors in the spread of antimicrobial resistance". Environmental Health and Preventive Medicine. 20 (4): 243–252. Bibcode:2015EHPM...20..243F. doi:10.1007/s12199-015-0468-0. PMC 4491066. PMID 25921603.
- ^ a b c d e f Ahmad I, Malak HA, Abulreesh HH (December 2021). "Environmental antimicrobial resistance and its drivers: a potential threat to public health". Journal of Global Antimicrobial Resistance. 27: 101–111. doi:10.1016/j.jgar.2021.08.001. PMID 34454098.
- ^ Monger XC, Gilbert AA, Saucier L, Vincent AT (October 2021). "Antibiotic Resistance: From Pig to Meat". Antibiotics. 10 (10): 1209. doi:10.3390/antibiotics10101209. PMC 8532907. PMID 34680790.
- ^ Torrella K (8 January 2023). "Big Meat just can't quit antibiotics". Vox. Archived from the original on 23 January 2023. Retrieved 23 January 2023.
- ^ a b Martin MJ, Thottathil SE, Newman TB (December 2015). "Antibiotics Overuse in Animal Agriculture: A Call to Action for Health Care Providers". American Journal of Public Health. 105 (12): 2409–2410. doi:10.2105/AJPH.2015.302870. PMC 4638249. PMID 26469675.
- ^ "Farm antibiotic use". saveourantibiotics.org. Archived from the original on 3 April 2024. Retrieved 21 March 2024.
- ^ Tang KL, Caffrey NP, Nóbrega DB, Cork SC, Ronksley PE, Barkema HW, et al. (November 2017). "Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis". The Lancet. Planetary Health. 1 (8): e316–e327. doi:10.1016/S2542-5196(17)30141-9. PMC 5785333. PMID 29387833.
- ^ a b Innes GK, Randad PR, Korinek A, Davis MF, Price LB, So AD, et al. (April 2020). "External Societal Costs of Antimicrobial Resistance in Humans Attributable to Antimicrobial Use in Livestock". Annual Review of Public Health. 41 (1): 141–157. doi:10.1146/annurev-publhealth-040218-043954. PMC 7199423. PMID 31910712.
- ^ "Veterinary feed directive (VFD) basics | American Veterinary Medical Association". avma.org. Archived from the original on 24 April 2024. Retrieved 24 April 2024.
- ^ US EPA O (15 March 2013). "What are Antimicrobial Pesticides?". US EPA. Archived from the original on 27 November 2022. Retrieved 28 February 2020.
- ^ Ramakrishnan B, Venkateswarlu K, Sethunathan N, Megharaj M (March 2019). "Local applications but global implications: Can pesticides drive microorganisms to develop antimicrobial resistance?". The Science of the Total Environment. 654: 177–189. Bibcode:2019ScTEn.654..177R. doi:10.1016/j.scitotenv.2018.11.041. PMID 30445319. S2CID 53568193.
- ^ Rhodes J, Abdolrasouli A, Dunne K, Sewell TR, Zhang Y, Ballard E, et al. (May 2022). "Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment". Nature Microbiology. 7 (5): 663–674. doi:10.1038/s41564-022-01091-2. PMC 9064804. PMID 35469019.
- ^ a b Verweij PE, Arendrup MC, Alastruey-Izquierdo A, Gold JA, Lockhart SR, Chiller T, et al. (December 2022). "Dual use of antifungals in medicine and agriculture: How do we help prevent resistance developing in human pathogens?". Drug Resistance Updates. 65: 100885. doi:10.1016/j.drup.2022.100885. PMC 10693676. PMID 36283187. S2CID 253052170.
- ^ a b Perron GG, Whyte L, Turnbaugh PJ, Goordial J, Hanage WP, Dantas G, et al. (25 March 2015). "Functional characterization of bacteria isolated from ancient arctic soil exposes diverse resistance mechanisms to modern antibiotics". PLOS ONE. 10 (3): e0069533. Bibcode:2015PLoSO..1069533P. doi:10.1371/journal.pone.0069533. PMC 4373940. PMID 25807523.
- ^ McGee D, Gribkoff E (4 August 2022). "Permafrost". MIT Climate Portal. Archived from the original on 27 September 2023. Retrieved 27 September 2023.
- ^ Fox-Kemper, B., H.T. Hewitt, C. Xiao, G. Aðalgeirsdóttir, S.S. Drijfhout, T.L. Edwards, N.R. Golledge, M. Hemer, R.E. Kopp, G. Krinner, A. Mix, D. Notz, S. Nowicki, I.S. Nurhati, L. Ruiz, J.-B. Sallée, A.B.A. Slangen, and Y. Yu, 2021: Chapter 9: Ocean, Cryosphere and Sea Level Change Archived 24 October 2022 at the Wayback Machine. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 1211–1362, doi:10.1017/9781009157896.011.
- ^ Michaeleen D. "Are There Zombie Viruses — Like The 1918 Flu — Thawing In The Permafrost?". NPR.org. Archived from the original on 24 April 2023. Retrieved 4 April 2023.
- ^ "Anthrax Outbreak In Russia Thought To Be Result Of Thawing Permafrost". NPR.org. Archived from the original on 22 September 2016. Retrieved 24 September 2016.
- ^ Ng TF, Chen LF, Zhou Y, Shapiro B, Stiller M, Heintzman PD, et al. (November 2014). "Preservation of viral genomes in 700-y-old caribou feces from a subarctic ice patch". Proceedings of the National Academy of Sciences of the United States of America. 111 (47): 16842–16847. Bibcode:2014PNAS..11116842N. doi:10.1073/pnas.1410429111. PMC 4250163. PMID 25349412.
- ^ Legendre M, Lartigue A, Bertaux L, Jeudy S, Bartoli J, Lescot M, et al. (September 2015). "In-depth study of Mollivirus sibericum, a new 30,000-y-old giant virus infecting Acanthamoeba". Proceedings of the National Academy of Sciences of the United States of America. 112 (38): E5327–E5335. Bibcode:2015PNAS..112E5327L. doi:10.1073/pnas.1510795112. JSTOR 26465169. PMC 4586845. PMID 26351664.
- ^ Alempic JM, Lartigue A, Goncharov AE, Grosse G, Strauss J, Tikhonov AN, et al. (February 2023). "An Update on Eukaryotic Viruses Revived from Ancient Permafrost". Viruses. 15 (2): 564. doi:10.3390/v15020564. PMC 9958942. PMID 36851778.
- ^ Alund NN (9 March 2023). "Scientists revive 'zombie virus' that was frozen for nearly 50,000 years". USA Today. Archived from the original on 24 April 2023. Retrieved 23 April 2023.
- ^ Sajjad W, Rafiq M, Din G, Hasan F, Iqbal A, Zada S, et al. (September 2020). "Resurrection of inactive microbes and resistome present in the natural frozen world: Reality or myth?". The Science of the Total Environment. 735: 139275. Bibcode:2020ScTEn.73539275S. doi:10.1016/j.scitotenv.2020.139275. PMID 32480145. S2CID 219169932.
- ^ Miner KR, D'Andrilli J, Mackelprang R, Edwards A, Malaska MJ, Waldrop MP, et al. (30 September 2021). "Emergent biogeochemical risks from Arctic permafrost degradation". Nature Climate Change. 11 (1): 809–819. Bibcode:2021NatCC..11..809M. doi:10.1038/s41558-021-01162-y. S2CID 238234156.
- ^ Wu R, Trubl G, Taş N, Jansson JK (15 April 2022). "Permafrost as a potential pathogen reservoir". One Earth. 5 (4): 351–360. Bibcode:2022OEart...5..351W. doi:10.1016/j.oneear.2022.03.010. S2CID 248208195.
- ^ Rogers Van Katwyk S, Giubilini A, Kirchhelle C, Weldon I, Harrison M, McLean A, et al. (March 2023). "Exploring Models for an International Legal Agreement on the Global Antimicrobial Commons: Lessons from Climate Agreements". Health Care Analysis. 31 (1): 25–46. doi:10.1007/s10728-019-00389-3. PMC 10042908. PMID 31965398.
- ^ Wilson LA, Van Katwyk SR, Weldon I, Hoffman SJ (2021). "A Global Pandemic Treaty Must Address Antimicrobial Resistance". The Journal of Law, Medicine & Ethics. 49 (4): 688–691. doi:10.1017/jme.2021.94. PMC 8749967. PMID 35006051.
- ^ a b c Spurling GK, Dooley L, Clark J, Askew DA, et al. (Cochrane Acute Respiratory Infections Group) (October 2023). "Immediate versus delayed versus no antibiotics for respiratory infections". The Cochrane Database of Systematic Reviews. 2023 (10): CD004417. doi:10.1002/14651858.CD004417.pub6. PMC 10548498. PMID 37791590.
- ^ a b c d "Duration of antibiotic therapy and resistance". NPS Medicinewise. National Prescribing Service Limited trading, Australia. 13 June 2013. Archived from the original on 23 July 2015. Retrieved 22 July 2015.
- ^ Abdelsalam Elshenawy R, Umaru N, Aslanpour Z (March 2024). "Shorter and Longer Antibiotic Durations for Respiratory Infections: To Fight Antimicrobial Resistance-A Retrospective Cross-Sectional Study in a Secondary Care Setting in the UK". Pharmaceuticals. 17 (3): 339. doi:10.3390/ph17030339. PMC 10975983. PMID 38543125.
- ^ a b c "Biggest Threats – Antibiotic/Antimicrobial Resistance – CDC". cdc.gov. 10 September 2018. Archived from the original on 12 September 2017. Retrieved 5 May 2016.
- ^ "HealthMap Resistance". HealthMap.org Boston Children's Hospital. Archived from the original on 15 November 2017. Retrieved 15 November 2017.
- ^ Scales D. "Mapping Antibiotic Resistance: Know The Germs in Your Neighborhood". WBUR. National Public Radio. Archived from the original on 8 December 2015. Retrieved 8 December 2015.
- ^ "ResistanceMap". Center for Disease Dynamics, Economics & Policy. Archived from the original on 14 November 2017. Retrieved 14 November 2017.
- ^ a b "Global Action Plan on Antimicrobial Resistance" (PDF). WHO. Archived from the original (PDF) on 31 October 2017. Retrieved 14 November 2017.
- ^ Mader R, Damborg P, Amat JP, Bengtsson B, Bourély C, Broens EM, et al. (January 2021). "Building the European Antimicrobial Resistance Surveillance network in veterinary medicine (EARS-Vet)". Euro Surveillance. 26 (4). doi:10.2807/1560-7917.ES.2021.26.4.2001359. PMC 7848785. PMID 33509339.
- ^ Feuer L, Frenzer SK, Merle R, Leistner R, Bäumer W, Bethe A, et al. (September 2024). "Prevalence of MRSA in canine and feline clinical samples from one-third of veterinary practices in Germany from 2019-2021". The Journal of Antimicrobial Chemotherapy. 79 (9): 2273–2280. doi:10.1093/jac/dkae225. PMID 39007221.
- ^ a b c d e f g Fisher MC, Alastruey-Izquierdo A, Berman J, Bicanic T, Bignell EM, Bowyer P, et al. (September 2022). "Tackling the emerging threat of antifungal resistance to human health". Nature Reviews. Microbiology. 20 (9): 557–571. doi:10.1038/s41579-022-00720-1. PMC 8962932. PMID 35352028.
- ^ Baur D, Gladstone BP, Burkert F, Carrara E, Foschi F, Döbele S, et al. (September 2017). "Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis". The Lancet. Infectious Diseases. 17 (9): 990–1001. doi:10.1016/S1473-3099(17)30325-0. PMID 28629876.
- ^ Gallagher JC, Justo JA, Chahine EB, Bookstaver PB, Scheetz M, Suda KJ, et al. (August 2018). "Preventing the Post-Antibiotic Era by Training Future Pharmacists as Antimicrobial Stewards". American Journal of Pharmaceutical Education. 82 (6): 6770. doi:10.5688/ajpe6770. PMC 6116871. PMID 30181677.
- ^ Andersson DI, Hughes D (September 2011). "Persistence of antibiotic resistance in bacterial populations". FEMS Microbiology Reviews. 35 (5): 901–11. doi:10.1111/j.1574-6976.2011.00289.x. PMID 21707669.
- ^ Gilberg K, Laouri M, Wade S, Isonaka S (2003). "Analysis of medication use patterns:apparent overuse of antibiotics and underuse of prescription drugs for asthma, depression, and CHF". Journal of Managed Care Pharmacy. 9 (3): 232–7. doi:10.18553/jmcp.2003.9.3.232. PMC 10437266. PMID 14613466. S2CID 25457069.
- ^ Llor C, Bjerrum L (December 2014). "Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem". Therapeutic Advances in Drug Safety. 5 (6): 229–41. doi:10.1177/2042098614554919. PMC 4232501. PMID 25436105.
- ^ "Pandemic of Antibiotic Resistance Killing Children in Bangladesh". Science Trend. 18 July 2021. Archived from the original on 29 November 2021. Retrieved 15 August 2021.
- ^ Chisti MJ, Harris JB, Carroll RW, Shahunja KM, Shahid AS, Moschovis PP, et al. (July 2021). "Antibiotic-Resistant Bacteremia in Young Children Hospitalized With Pneumonia in Bangladesh Is Associated With a High Mortality Rate". Open Forum Infectious Diseases. 8 (7): ofab260. doi:10.1093/ofid/ofab260. PMC 8280371. PMID 34277885.
- ^ The WHO AWaRe (Access, Watch, Reserve) antibiotic book. Geneva: World Health Organization (WHO). 2022. ISBN 978-92-4-006238-2. Archived from the original on 13 August 2023. Retrieved 28 March 2023.
- ^ Talento AF, Qualie M, Cottom L, Backx M, White PL (September 2021). "Lessons from an Educational Invasive Fungal Disease Conference on Hospital Antifungal Stewardship Practices across the UK and Ireland". Journal of Fungi. 7 (10): 801. doi:10.3390/jof7100801. PMC 8538376. PMID 34682223.
- ^ Doron S, Davidson LE (November 2011). "Antimicrobial stewardship". Mayo Clinic Proceedings. 86 (11): 1113–23. doi:10.4065/mcp.2011.0358. PMC 3203003. PMID 22033257.
- ^ Davey P, Marwick CA, Scott CL, Charani E, McNeil K, Brown E, et al. (February 2017). "Interventions to improve antibiotic prescribing practices for hospital inpatients". The Cochrane Database of Systematic Reviews. 2017 (2): CD003543. doi:10.1002/14651858.cd003543.pub4. PMC 6464541. PMID 28178770.
- ^ a b Costa T, Pimentel AC, Mota-Vieira L, Castanha AC (1 May 2021). "The benefits of a unit dose system in oral antibiotics dispensing: Azorean hospital pharmacists tackling the socioeconomic problem of leftovers in Portugal". Drugs & Therapy Perspectives. 37 (5): 212–221. doi:10.1007/s40267-021-00825-2. ISSN 1179-1977.
- ^ O'Sullivan JW, Harvey RT, Glasziou PP, McCullough A (November 2016). "Written information for patients (or parents of child patients) to reduce the use of antibiotics for acute upper respiratory tract infections in primary care". The Cochrane Database of Systematic Reviews. 2016 (11): CD011360. doi:10.1002/14651858.CD011360.pub2. PMC 6464519. PMID 27886368.
- ^ Bosley H, Henshall C, Appleton JV, Jackson D (March 2018). "A systematic review to explore influences on parental attitudes towards antibiotic prescribing in children" (PDF). Journal of Clinical Nursing. 27 (5–6): 892–905. doi:10.1111/jocn.14073. PMID 28906047. S2CID 4336064. Archived (PDF) from the original on 14 October 2023. Retrieved 6 May 2023.
- ^ "The Five Rights of Medication Administration". ihi.org. March 2007. Archived from the original on 24 October 2015. Retrieved 30 October 2015.
- ^ "CDC Features – Mission Critical: Preventing Antibiotic Resistance". cdc.gov. 4 April 2018. Archived from the original on 8 November 2017. Retrieved 22 July 2015.
- ^ Leekha S, Terrell CL, Edson RS (February 2011). "General principles of antimicrobial therapy". Mayo Clinic Proceedings. 86 (2): 156–67. doi:10.4065/mcp.2010.0639. PMC 3031442. PMID 21282489. Archived from the original on 15 September 2019. Retrieved 22 July 2015.
- ^ a b "Are you using antibiotics wisely?". Centers for Disease Control and Prevention. 3 January 2022. Archived from the original on 21 March 2024. Retrieved 21 March 2024.
- ^ "Articles". Cedars-Sinai. Archived from the original on 30 May 2020. Retrieved 23 March 2024.
- ^ "Defined Daily Dose (DDD)". WHO. Archived from the original on 10 May 2023. Retrieved 28 March 2023.
- ^ "Indicator: Antibiotic prescribing". QualityWatch. Nuffield Trust & Health Foundation. Archived from the original on 14 January 2015. Retrieved 16 July 2015.
- ^ a b c IACG (2018) Reduce unintentional exposure and the need for antimicrobials, and optimize their use IACG Discussion Paper Archived 6 July 2021 at the Wayback Machine, Interagency Coordination Group on Antimicrobial Resistance, public consultation process at WHO, Geneva, Switzerland
- ^ a b c d e Araya P (May 2016). "The Impact of Water and Sanitation on Diarrhoeal Disease Burden and Over-Consumption of Anitbiotics" (PDF). Archived (PDF) from the original on 1 October 2017. Retrieved 12 November 2017.
- ^ "Goal 6: Ensure availability and sustainable management of water and sanitation for all". United Nations Department of Economic and Social Affairs. Archived from the original on 24 September 2020. Retrieved 17 April 2023.
- ^ Swoboda SM, Earsing K, Strauss K, Lane S, Lipsett PA (February 2004). "Electronic monitoring and voice prompts improve hand hygiene and decrease nosocomial infections in an intermediate care unit". Critical Care Medicine. 32 (2): 358–63. doi:10.1097/01.CCM.0000108866.48795.0F. PMID 14758148. S2CID 9817602.(subscription required)
- ^ WHO, UNICEF (2015). Water, sanitation and hygiene in health care facilities – Status in low and middle income countries and way forward Archived 12 September 2018 at the Wayback Machine. World Health Organization (WHO), Geneva, Switzerland, ISBN 978 92 4 150847 6
- ^ Agga GE, Schmidt JW, Arthur TM (December 2016). "Effects of In-Feed Chlortetracycline Prophylaxis in Beef Cattle on Animal Health and Antimicrobial-Resistant Escherichia coli". Applied and Environmental Microbiology. 82 (24): 7197–7204. Bibcode:2016ApEnM..82.7197A. doi:10.1128/AEM.01928-16. PMC 5118930. PMID 27736789.
- ^ a b Brown EE, Cooper A, Carrillo C, Blais B (2019). "Selection of Multidrug-Resistant Bacteria in Medicated Animal Feeds". Frontiers in Microbiology. 10: 456. doi:10.3389/fmicb.2019.00456. PMC 6414793. PMID 30894847.
- ^ Marshall BM, Levy SB (October 2011). "Food animals and antimicrobials: impacts on human health". Clinical Microbiology Reviews. 24 (4): 718–33. doi:10.1128/CMR.00002-11. PMC 3194830. PMID 21976606.
- ^ Casewell M, Friis C, Marco E, McMullin P, Phillips I (August 2003). "The European ban on growth-promoting antibiotics and emerging consequences for human and animal health". The Journal of Antimicrobial Chemotherapy. 52 (2): 159–61. doi:10.1093/jac/dkg313. PMID 12837737.
- ^ Castanon JI (November 2007). "History of the use of antibiotic as growth promoters in European poultry feeds". Poultry Science. 86 (11): 2466–71. doi:10.3382/ps.2007-00249. PMID 17954599.(subscription required)
- ^ Bengtsson B, Wierup M (2006). "Antimicrobial resistance in Scandinavia after ban of antimicrobial growth promoters". Animal Biotechnology. 17 (2): 147–56. doi:10.1080/10495390600956920. PMID 17127526. S2CID 34602891.(subscription required)
- ^ Angulo FJ, Baker NL, Olsen SJ, Anderson A, Barrett TJ (April 2004). "Antimicrobial use in agriculture: controlling the transfer of antimicrobial resistance to humans". Seminars in Pediatric Infectious Diseases. 15 (2): 78–85. doi:10.1053/j.spid.2004.01.010. PMID 15185190.
- ^ "GAO-11-801, Antibiotic Resistance: Agencies Have Made Limited Progress Addressing Antibiotic Use in Animals". gao.gov. Archived from the original on 5 November 2013. Retrieved 25 January 2014.
- ^ Nelson JM, Chiller TM, Powers JH, Angulo FJ (April 2007). "Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story". Clinical Infectious Diseases. 44 (7): 977–80. doi:10.1086/512369. PMID 17342653.
- ^ "Extralabel Use and Antimicrobials". FDA. 29 April 2022. Archived from the original on 19 April 2023. Retrieved 19 April 2023.
- ^ Pallo-Zimmerman LM, Byron JK, Graves TK (July 2010). "Fluoroquinolones: then and now" (PDF). Compendium. 32 (7): E1-9, quiz E9. PMID 20957609. Archived (PDF) from the original on 21 June 2023. Retrieved 19 April 2023.
- ^ "RAND Europe Focus on Antimicrobial Resistance (AMR)". rand.org. Archived from the original on 21 April 2018. Retrieved 23 April 2018.
- ^ "React". Archived from the original on 16 November 2017. Retrieved 16 November 2017.
- ^ "Antibiotic Resistance: the silent tsunami (youtube video)". ReActTube. 6 March 2017. Archived from the original on 11 November 2021. Retrieved 17 November 2017.
- ^ "The Antibiotic Apocalypse Explained". Kurzgesagt – In a Nutshell. 16 March 2016. Archived from the original on 20 March 2021. Retrieved 17 November 2017.
- ^ "Ireland's National Action Plan on Antimicrobial Resistance 2017 – 2020". October 2017. Archived from the original on 10 August 2022. Retrieved 11 January 2019 – via Lenus (Irish Health Repository).
- ^ SARI Hospital Antimicrobial Stewardship Working Group (2009). Guidelines for antimicrobial stewardship in hospitals in Ireland. Dublin: HSE Health Protection Surveillance Centre (HPSC). ISBN 978-0-9551236-7-2. Archived from the original on 5 December 2021. Retrieved 11 January 2019.
- ^ "Taking antibiotics for colds and flu? There's no point". HSE.ie. Archived from the original on 28 May 2018. Retrieved 11 January 2019.
- ^ Murphy M, Bradley CP, Byrne S (May 2012). "Antibiotic prescribing in primary care, adherence to guidelines and unnecessary prescribing—an Irish perspective". BMC Family Practice. 13 (1): 43. doi:10.1186/1471-2296-13-43. PMC 3430589. PMID 22640399.
- ^ "UK 20-year vision for antimicrobial resistance". GOV.UK. Archived from the original on 28 March 2023. Retrieved 28 March 2023.
- ^ "UK 5-year action plan for antimicrobial resistance 2019 to 2024". GOV.UK. Archived from the original on 28 March 2023. Retrieved 28 March 2023.
- ^ "WHO bacterial priority pathogens list, 2024: Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance". who.int. Archived from the original on 20 May 2024. Retrieved 20 May 2024.
- ^ "World Antibiotic Awareness Week". World Health Organization. Archived from the original on 20 November 2015. Retrieved 20 November 2015.
- ^ "World Antibiotic Awareness Week". WHO. Archived from the original on 13 November 2017. Retrieved 14 November 2017.
- ^ a b "WHO | UN Interagency Coordination Group (IACG) on Antimicrobial Resistance". WHO. Archived from the original on 20 March 2017. Retrieved 7 August 2019.
- ^ Interagency Coordination Group (IACG) (April 2019). No Time to Wait: Securing the Future from Drug-Resistant Infections (PDF) (Report). Archived (PDF) from the original on 19 April 2023. Retrieved 19 April 2023.
- ^ Reygaert WC (2018). "An overview of the antimicrobial resistance mechanisms of bacteria". AIMS Microbiology. 4 (3): 482–501. doi:10.3934/microbiol.2018.3.482. PMC 6604941. PMID 31294229.
- ^ Baylay AJ, Piddock LJ, Webber MA (14 March 2019). "Molecular Mechanisms of Antibiotic Resistance – Part I". Bacterial Resistance to Antibiotics – from Molecules to Man. Wiley. pp. 1–26. doi:10.1002/9781119593522.ch1. ISBN 978-1-119-94077-7. S2CID 202806156.
- ^ Connell SR, Tracz DM, Nierhaus KH, Taylor DE (December 2003). "Ribosomal protection proteins and their mechanism of tetracycline resistance". Antimicrobial Agents and Chemotherapy. 47 (12): 3675–81. doi:10.1128/AAC.47.12.3675-3681.2003. PMC 296194. PMID 14638464.
- ^ Henry RJ (December 1943). "The Mode of Action of Sulfonamides". Bacteriological Reviews. 7 (4): 175–262. doi:10.1128/MMBR.7.4.175-262.1943. PMC 440870. PMID 16350088.
- ^ Li XZ, Nikaido H (August 2009). "Efflux-mediated drug resistance in bacteria: an update". Drugs. 69 (12): 1555–623. doi:10.2165/11317030-000000000-00000. PMC 2847397. PMID 19678712.
- ^ Aminov RI, Mackie RI (June 2007). "Evolution and ecology of antibiotic resistance genes". FEMS Microbiology Letters. 271 (2): 147–61. doi:10.1111/j.1574-6968.2007.00757.x. PMID 17490428.
- ^ Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, et al. (July 1998). "NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli". Antimicrobial Agents and Chemotherapy. 42 (7): 1778–82. doi:10.1128/AAC.42.7.1778. PMC 105682. PMID 9661020.
- ^ Duval M, Dar D, Carvalho F, Rocha EP, Sorek R, Cossart P (December 2018). "HflXr, a homolog of a ribosome-splitting factor, mediates antibiotic resistance". Proceedings of the National Academy of Sciences of the United States of America. 115 (52): 13359–13364. Bibcode:2018PNAS..11513359D. doi:10.1073/pnas.1810555115. PMC 6310831. PMID 30545912.
- ^ "About Antibiotic Resistance". CDC. 13 March 2020. Archived from the original on 1 October 2017. Retrieved 8 September 2017.
- ^ Robicsek A, Jacoby GA, Hooper DC (October 2006). "The worldwide emergence of plasmid-mediated quinolone resistance". The Lancet. Infectious Diseases. 6 (10): 629–40. doi:10.1016/S1473-3099(06)70599-0. PMID 17008172.
- ^ Ochiai K, Yamanaka T, Kimura K, Sawada O, O (1959). "Inheritance of drug resistance (and its transfer) between Shigella strains and Between Shigella and E.coli strains". Hihon Iji Shimpor (in Japanese). 34: 1861.
- ^ Watford S, Warrington SJ (2018). "Bacterial DNA Mutations". StatPearls. StatPearls Publishing. PMID 29083710. Archived from the original on 8 March 2021. Retrieved 21 January 2019.
- ^ Levin BR, Perrot V, Walker N (March 2000). "Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria". Genetics. 154 (3): 985–97. doi:10.1093/genetics/154.3.985. PMC 1460977. PMID 10757748. Archived from the original on 18 January 2023. Retrieved 4 March 2017.
- ^ Hotchkiss RD (1951). "Transfer of penicillin resistance in pneumococci by the desoxyribonucleate derived from resistant cultures". Cold Spring Harbor Symposia on Quantitative Biology. 16: 457–61. doi:10.1101/SQB.1951.016.01.032. PMID 14942755.
- ^ Ubukata K, Konno M, Fujii R (September 1975). "Transduction of drug resistance to tetracycline, chloramphenicol, macrolides, lincomycin and clindamycin with phages induced from Streptococcus pyogenes". The Journal of Antibiotics. 28 (9): 681–8. doi:10.7164/antibiotics.28.681. PMID 1102514.
- ^ von Wintersdorff CJ, Penders J, van Niekerk JM, Mills ND, Majumder S, van Alphen LB, et al. (19 February 2016). "Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer". Frontiers in Microbiology. 7: 173. doi:10.3389/fmicb.2016.00173. PMC 4759269. PMID 26925045.
- ^ Chan CX, Beiko RG, Ragan MA (August 2011). "Lateral transfer of genes and gene fragments in Staphylococcus extends beyond mobile elements". Journal of Bacteriology. 193 (15): 3964–77. doi:10.1128/JB.01524-10. PMC 3147504. PMID 21622749.
- ^ Johansen TB, Scheffer L, Jensen VK, Bohlin J, Feruglio SL (June 2018). "Whole-genome sequencing and antimicrobial resistance in Brucella melitensis from a Norwegian perspective". Scientific Reports. 8 (1): 8538. Bibcode:2018NatSR...8.8538J. doi:10.1038/s41598-018-26906-3. PMC 5986768. PMID 29867163.
- ^ Tirumalai MR, Karouia F, Tran Q, Stepanov VG, Bruce RJ, Ott M, et al. (January 2019). "Evaluation of acquired antibiotic resistance in Escherichia coli exposed to long-term low-shear modeled microgravity and background antibiotic exposure". mBio. 10 (e02637-18). doi:10.1128/mBio.02637-18. PMC 6336426. PMID 30647159.
- ^ Tirumalai MR, Karouia F, Tran Q, Stepanov VG, Bruce RJ, Ott M, et al. (May 2017). "The adaptation of Escherichia coli cells grown in simulated microgravity for an extended period is both phenotypic and genomic". npj Microgravity. 3 (15): 15. doi:10.1038/s41526-017-0020-1. PMC 5460176. PMID 28649637.
- ^ Ghaith DM, Mohamed ZK, Farahat MG, Aboulkasem Shahin W, Mohamed HO (March 2019). "Colonization of intestinal microbiota with carbapenemase-producing Enterobacteriaceae in paediatric intensive care units in Cairo, Egypt". Arab Journal of Gastroenterology. 20 (1): 19–22. doi:10.1016/j.ajg.2019.01.002. PMID 30733176. S2CID 73444389. Archived from the original on 27 November 2022. Retrieved 30 May 2022.
- ^ Diene SM, Rolain JM (September 2014). "Carbapenemase genes and genetic platforms in Gram-negative bacilli: Enterobacteriaceae, Pseudomonas and Acinetobacter species". Clinical Microbiology and Infection. 20 (9): 831–8. doi:10.1111/1469-0691.12655. PMID 24766097.
- ^ Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. (September 2010). "Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study". The Lancet. Infectious Diseases. 10 (9): 597–602. doi:10.1016/S1473-3099(10)70143-2. PMC 2933358. PMID 20705517.
- ^ Hudson CM, Bent ZW, Meagher RJ, Williams KP (7 June 2014). "Resistance determinants and mobile genetic elements of an NDM-1-encoding Klebsiella pneumoniae strain". PLOS ONE. 9 (6): e99209. Bibcode:2014PLoSO...999209H. doi:10.1371/journal.pone.0099209. PMC 4048246. PMID 24905728.
- ^ Lou Z, Sun Y, Rao Z (February 2014). "Current progress in antiviral strategies". Trends in Pharmacological Sciences. 35 (2): 86–102. doi:10.1016/j.tips.2013.11.006. PMC 7112804. PMID 24439476.
- ^ Pennings PS (June 2013). "HIV Drug Resistance: Problems and Perspectives". Infectious Disease Reports. 5 (Suppl 1): e5. doi:10.4081/idr.2013.s1.e5. PMC 3892620. PMID 24470969.
- ^ Das K, Arnold E (April 2013). "HIV-1 reverse transcriptase and antiviral drug resistance. Part 1". Current Opinion in Virology. 3 (2): 111–8. doi:10.1016/j.coviro.2013.03.012. PMC 4097814. PMID 23602471.
- ^ Ton Q, Frenkel L (March 2013). "HIV drug resistance in mothers and infants following use of antiretrovirals to prevent mother-to-child transmission". Current HIV Research. 11 (2): 126–36. doi:10.2174/1570162x11311020005. PMID 23432488.
- ^ Ebrahim O, Mazanderani AH (June 2013). "Recent developments in hiv treatment and their dissemination in poor countries". Infectious Disease Reports. 5 (Suppl 1): e2. doi:10.4081/idr.2013.s1.e2. PMC 3892621. PMID 24470966.
- ^ Xie JL, Polvi EJ, Shekhar-Guturja T, Cowen LE (2014). "Elucidating drug resistance in human fungal pathogens". Future Microbiology. 9 (4): 523–42. doi:10.2217/fmb.14.18. PMID 24810351.
- ^ Srinivasan A, Lopez-Ribot JL, Ramasubramanian AK (March 2014). "Overcoming antifungal resistance". Drug Discovery Today: Technologies. 11: 65–71. doi:10.1016/j.ddtec.2014.02.005. PMC 4031462. PMID 24847655.
- ^ Costa C, Dias PJ, Sá-Correia I, Teixeira MC (2014). "MFS multidrug transporters in pathogenic fungi: do they have real clinical impact?". Frontiers in Physiology. 5: 197. doi:10.3389/fphys.2014.00197. PMC 4035561. PMID 24904431.
- ^ World Health Organization, ed. (25 October 2022). WHO fungal priority pathogens list to guide research, development and public health action. World Health Organization. ISBN 978-92-4-006024-1. Archived from the original on 26 October 2022. Retrieved 27 October 2022.
- ^ a b Andrews KT, Fisher G, Skinner-Adams TS (August 2014). "Drug repurposing and human parasitic protozoan diseases". International Journal for Parasitology: Drugs and Drug Resistance. 4 (2): 95–111. doi:10.1016/j.ijpddr.2014.02.002. PMC 4095053. PMID 25057459.
- ^ Visser BJ, van Vugt M, Grobusch MP (October 2014). "Malaria: an update on current chemotherapy". Expert Opinion on Pharmacotherapy. 15 (15): 2219–54. doi:10.1517/14656566.2014.944499. PMID 25110058. S2CID 34991324.
- ^ Chia WN, Goh YS, Rénia L (2014). "Novel approaches to identify protective malaria vaccine candidates". Frontiers in Microbiology. 5: 586. doi:10.3389/fmicb.2014.00586. PMC 4233905. PMID 25452745.
- ^ Franco JR, Simarro PP, Diarra A, Jannin JG (2014). "Epidemiology of human African trypanosomiasis". Clinical Epidemiology. 6: 257–75. doi:10.2147/CLEP.S39728. PMC 4130665. PMID 25125985.
- ^ Herrera L (2014). "Trypanosoma cruzi, the Causal Agent of Chagas Disease: Boundaries between Wild and Domestic Cycles in Venezuela". Frontiers in Public Health. 2: 259. doi:10.3389/fpubh.2014.00259. PMC 4246568. PMID 25506587.
- ^ Mansueto P, Seidita A, Vitale G, Cascio A (2014). "Leishmaniasis in travelers: a literature review" (PDF). Travel Medicine and Infectious Disease. 12 (6 Pt A): 563–81. doi:10.1016/j.tmaid.2014.09.007. hdl:10447/101959. PMID 25287721. Archived (PDF) from the original on 12 October 2022. Retrieved 23 October 2017.
- ^ a b c Munk P, Brinch C, Møller FD, Petersen TN, Hendriksen RS, Seyfarth AM, et al. (December 2022). "Genomic analysis of sewage from 101 countries reveals global landscape of antimicrobial resistance". Nature Communications. 13 (1): 7251. Bibcode:2022NatCo..13.7251M. doi:10.1038/s41467-022-34312-7. PMC 9715550. PMID 36456547.
- ^ "Antibiotika-Resistenzen verbreiten sich offenbar anders als gedacht". Deutschlandfunk Nova (in German). Archived from the original on 17 January 2023. Retrieved 17 January 2023.
- ^ "Superbugs on the rise: WHO report signals increase in antibiotic resistance". World Health Organization via medicalxpress.com. Archived from the original on 2 February 2023. Retrieved 18 January 2023.
- ^ "Global antimicrobial resistance and use surveillance system (GLASS) report: 2022". who.int. Archived from the original on 21 January 2023. Retrieved 18 January 2023.
- ^ "Patients contracted 165 antibiotic resistant infections each day in 2018, says PHE". Pharmaceutical Journal. 31 October 2019. Archived from the original on 26 July 2020. Retrieved 11 December 2019.
- ^ "National Infection & Death Estimates for AR". Centers for Disease Control and Prevention. 6 July 2022. Archived from the original on 3 August 2023. Retrieved 3 August 2023.
- ^ "COVID-19 & Antibiotic Resistance". Centers for Disease Control and Prevention. 11 August 2022. Archived from the original on 21 February 2022. Retrieved 3 August 2023.
- ^ "2022 SPECIAL REPORT: COVID-19 U.S. IMPACT ON ANTIMICROBIAL RESISTANCE" (PDF). Centers for Disease Control and Prevention. 2022. p. 7. Archived (PDF) from the original on 26 August 2023. Retrieved 3 August 2023.
- ^ Aminov RI (2010). "A brief history of the antibiotic era: lessons learned and challenges for the future". Frontiers in Microbiology. 1: 134. doi:10.3389/fmicb.2010.00134. PMC 3109405. PMID 21687759.
- ^ Carvalho G, Forestier C, Mathias JD (December 2019). "Antibiotic resilience: a necessary concept to complement antibiotic resistance?". Proceedings. Biological Sciences. 286 (1916): 20192408. doi:10.1098/rspb.2019.2408. PMC 6939251. PMID 31795866.
- ^ a b Antimicrobial resistance : global report on surveillance. Geneva, Switzerland: World Health Organization. 2014. ISBN 978-92-4-156474-8. OCLC 880847527.
- ^ Amábile-Cuevas CF, ed. (2007). Antimicrobial resistance in bacteria. Horizon Scientific Press.
- ^ Fleming A (11 December 1945). "Penicillin" (PDF). Nobel Lecture. Archived (PDF) from the original on 31 March 2018. Retrieved 9 August 2020.
- ^ "WHO | Global action plan on antimicrobial resistance". WHO. Archived from the original on 18 April 2018. Retrieved 23 April 2018.
- ^ a b Pollack A (20 January 2016). "To Fight 'Superbugs,' Drug Makers Call for Incentives to Develop Antibiotics". New York Times. Davos 2016 Special Report. Davos, Switzerland. Archived from the original on 24 April 2018. Retrieved 24 January 2016.
- ^ Leonard C, Crabb N, Glover D, Cooper S, Bouvy J, Wobbe M, et al. (May 2023). "Can the UK 'Netflix' Payment Model Boost the Antibacterial Pipeline?". Applied Health Economics and Health Policy. 21 (3): 365–372. doi:10.1007/s40258-022-00786-1. PMC 9842493. PMID 36646872.
- ^ a b Behdinan A, Hoffman SJ, Pearcey M (2015). "Some Global Policies for Antibiotic Resistance Depend on Legally Binding and Enforceable Commitments". The Journal of Law, Medicine & Ethics. 43 (2 Suppl 3): 68–73. doi:10.1111/jlme.12277. PMID 26243246. S2CID 7415203.
- ^ Hoffman SJ, Outterson K (2015). "What Will It Take to Address the Global Threat of Antibiotic Resistance?". The Journal of Law, Medicine & Ethics. 43 (2): 363–8. doi:10.1111/jlme.12253. PMID 26242959. S2CID 41987305. Archived from the original on 12 October 2022. Retrieved 11 November 2019.
- ^ a b c Hoffman SJ, Outterson K, Røttingen JA, Cars O, Clift C, Rizvi Z, et al. (February 2015). "An international legal framework to address antimicrobial resistance". Bulletin of the World Health Organization. 93 (2): 66. doi:10.2471/BLT.15.152710 (inactive 9 November 2024). PMC 4339972. PMID 25883395.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Rizvi Z, Hoffman SJ (2015). "Effective Global Action on Antibiotic Resistance Requires Careful Consideration of Convening Forums". The Journal of Law, Medicine & Ethics. 43 (2 Suppl 3): 74–8. doi:10.1111/jlme.12278. PMID 26243247. S2CID 24223063.
- ^ Andresen S, Hoffman SJ (2015). "Much Can Be Learned about Addressing Antibiotic Resistance from Multilateral Environmental Agreements". Journal of Law, Medicine & Ethics. 43 (2): 46–52.
- ^ President's 2016 Budget Proposes Historic Investment to Combat Antibiotic-Resistant Bacteria to Protect Public Health Archived 22 January 2017 at the Wayback Machine The White House, Office of the Press Secretary, 27 January 2015
- ^ a b "FACT SHEET: Obama Administration Releases National Action Plan to Combat Antibiotic-Resistant Bacteria". whitehouse.gov. 27 March 2015. Archived from the original on 21 January 2017. Retrieved 30 October 2015 – via National Archives.
- ^ a b "Antibiotic Resistance Threats in the United States, 2023". Centers for Disease Control and Prevention. Retrieved 20 October 2023.
- ^ Petrakis V, Panopoulou M, Rafailidis P, Lemonakis N, Lazaridis G, Terzi I, et al. (2023). "The Impact of the COVID-19 Pandemic on Antimicrobial Resistance and Management of Bloodstream Infections". Pathogens. 21 (6): e22–e25. doi:10.3390/pathogens12060780. PMC 10302285. PMID 37375470.
- ^ "Get Ahead of Sepsis". Centers for Disease Control and Prevention. Retrieved 20 October 2023.
- ^ "National Action Plan for Combating Antibiotic-Resistant Bacteria (CARB) 2023-2028". The White House. Retrieved 20 October 2023.
- ^ "PASTEUR Act of 2023". United States Congress. Retrieved 20 October 2023.
- ^ Knight GM, Davies NG, Colijn C, Coll F, Donker T, Gifford DR, et al. (November 2019). "Mathematical modelling for antibiotic resistance control policy: do we know enough?". BMC Infectious Diseases. 19 (1): 1011. doi:10.1186/s12879-019-4630-y. PMC 6884858. PMID 31783803.
- ^ Hopkins H, Bruxvoort KJ, Cairns ME, Chandler CI, Leurent B, Ansah EK, et al. (March 2017). "Impact of introduction of rapid diagnostic tests for malaria on antibiotic prescribing: analysis of observational and randomised studies in public and private healthcare settings". BMJ. 356: j1054. doi:10.1136/bmj.j1054. PMC 5370398. PMID 28356302.
- ^ "Diagnostics Are Helping Counter Antimicrobial Resistance, But More Work Is Needed". MDDI Online. 20 November 2018. Archived from the original on 2 December 2018. Retrieved 2 December 2018.
- ^ a b van Belkum A, Bachmann TT, Lüdke G, Lisby JG, Kahlmeter G, Mohess A, et al. (January 2019). "Developmental roadmap for antimicrobial susceptibility testing systems". Nature Reviews. Microbiology. 17 (1): 51–62. doi:10.1038/s41579-018-0098-9. hdl:2445/132505. PMC 7138758. PMID 30333569.
- ^ "Progress on antibiotic resistance". Nature. 562 (7727): 307. October 2018. Bibcode:2018Natur.562Q.307.. doi:10.1038/d41586-018-07031-7. PMID 30333595.
- ^ Kim JI, Maguire F, Tsang KK, Gouliouris T, Peacock SJ, McAllister TA, et al. (September 2022). "Machine Learning for Antimicrobial Resistance Prediction: Current Practice, Limitations, and Clinical Perspective". Clinical Microbiology Reviews. 35 (3): e0017921. doi:10.1128/cmr.00179-21. PMC 9491192. PMID 35612324.
- ^ Banerjee R, Patel R (February 2023). "Molecular diagnostics for genotypic detection of antibiotic resistance: current landscape and future directions". JAC-antimicrobial Resistance. 5 (1): dlad018. doi:10.1093/jacamr/dlad018. PMC 9937039. PMID 36816746.
- ^ Baltekin Ö, Boucharin A, Tano E, Andersson DI, Elf J (August 2017). "Antibiotic susceptibility testing in less than 30 min using direct single-cell imaging". Proceedings of the National Academy of Sciences of the United States of America. 114 (34): 9170–9175. Bibcode:2017PNAS..114.9170B. doi:10.1073/pnas.1708558114. PMC 5576829. PMID 28790187.
- ^ Luro S, Potvin-Trottier L, Okumus B, Paulsson J (January 2020). "Isolating live cells after high-throughput, long-term, time-lapse microscopy". Nature Methods. 17 (1): 93–100. doi:10.1038/s41592-019-0620-7. PMC 7525750. PMID 31768062.
- ^ Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, et al. (Cochrane Acute Respiratory Infections Group) (October 2017). "Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections". The Cochrane Database of Systematic Reviews. 10 (10): CD007498. doi:10.1002/14651858.CD007498.pub3. PMC 6485408. PMID 29025194.
- ^ Smedemark SA, Aabenhus R, Llor C, Fournaise A, Olsen O, Jørgensen KJ, et al. (Cochrane Acute Respiratory Infections Group) (October 2022). "Biomarkers as point-of-care tests to guide prescription of antibiotics in people with acute respiratory infections in primary care". The Cochrane Database of Systematic Reviews. 2022 (10): CD010130. doi:10.1002/14651858.CD010130.pub3. PMC 9575154. PMID 36250577.
- ^ Doan Q, Enarson P, Kissoon N, Klassen TP, Johnson DW (September 2014). "Rapid viral diagnosis for acute febrile respiratory illness in children in the Emergency Department". The Cochrane Database of Systematic Reviews. 2014 (9): CD006452. doi:10.1002/14651858.CD006452.pub4. PMC 6718218. PMID 25222468.
- ^ Mishra RP, Oviedo-Orta E, Prachi P, Rappuoli R, Bagnoli F (October 2012). "Vaccines and antibiotic resistance". Current Opinion in Microbiology. 15 (5): 596–602. doi:10.1016/j.mib.2012.08.002. PMID 22981392.
- ^ Kennedy DA, Read AF (March 2017). "Why does drug resistance readily evolve but vaccine resistance does not?". Proceedings. Biological Sciences. 284 (1851): 20162562. doi:10.1098/rspb.2016.2562. PMC 5378080. PMID 28356449.
- ^ Kennedy DA, Read AF (December 2018). "Why the evolution of vaccine resistance is less of a concern than the evolution of drug resistance". Proceedings of the National Academy of Sciences of the United States of America. 115 (51): 12878–12886. Bibcode:2018PNAS..11512878K. doi:10.1073/pnas.1717159115. PMC 6304978. PMID 30559199.
- ^ "Immunity, Infectious Diseases, and Pandemics—What You Can Do". HomesteadSchools.com. Archived from the original on 3 December 2013. Retrieved 12 June 2013.
- ^ Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, et al. (February 2002). "Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis". The New England Journal of Medicine. 346 (7): 491–6. doi:10.1056/NEJMoa011297. PMID 11844850.
- ^ a b Fowler VG, Proctor RA (May 2014). "Where does a Staphylococcus aureus vaccine stand?". Clinical Microbiology and Infection. 20 (5): 66–75. doi:10.1111/1469-0691.12570. PMC 4067250. PMID 24476315.
- ^ McNeely TB, Shah NA, Fridman A, Joshi A, Hartzel JS, Keshari RS, et al. (2014). "Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors". Human Vaccines & Immunotherapeutics. 10 (12): 3513–6. doi:10.4161/hv.34407. PMC 4514053. PMID 25483690.
- ^ Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, et al. (January 2020). "Development of therapeutic antibodies for the treatment of diseases". Journal of Biomedical Science. 27 (1): 1. doi:10.1186/s12929-019-0592-z. PMC 6939334. PMID 31894001.
- ^ a b c Singh G, Rana A (May 2024). "Decoding antimicrobial resistance: unraveling molecular mechanisms and targeted strategies". Archives of Microbiology. 206 (6): 280. Bibcode:2024ArMic.206..280S. doi:10.1007/s00203-024-03998-2. PMID 38805035.
- ^ Kim S, Lieberman TD, Kishony R (October 2014). "Alternating antibiotic treatments constrain evolutionary paths to multidrug resistance". Proceedings of the National Academy of Sciences of the United States of America. 111 (40): 14494–9. Bibcode:2014PNAS..11114494K. doi:10.1073/pnas.1409800111. PMC 4210010. PMID 25246554.
- ^ Pál C, Papp B, Lázár V (July 2015). "Collateral sensitivity of antibiotic-resistant microbes". Trends in Microbiology. 23 (7): 401–7. doi:10.1016/j.tim.2015.02.009. PMC 5958998. PMID 25818802.
- ^ Imamovic L, Ellabaan MM, Dantas Machado AM, Citterio L, Wulff T, Molin S, et al. (January 2018). "Drug-Driven Phenotypic Convergence Supports Rational Treatment Strategies of Chronic Infections". Cell. 172 (1–2): 121–134.e14. doi:10.1016/j.cell.2017.12.012. PMC 5766827. PMID 29307490.
- ^ Beckley AM, Wright ES (October 2021). "Identification of antibiotic pairs that evade concurrent resistance via a retrospective analysis of antimicrobial susceptibility test results". The Lancet. Microbe. 2 (10): e545–e554. doi:10.1016/S2666-5247(21)00118-X. PMC 8496867. PMID 34632433.
- ^ Ma Y, Chua SL (15 November 2021). "No collateral antibiotic sensitivity by alternating antibiotic pairs". The Lancet Microbe. 3 (1): e7. doi:10.1016/S2666-5247(21)00270-6. ISSN 2666-5247. PMID 35544116. S2CID 244147577.
- ^ Liu J, Bedell TA, West JG, Sorensen EJ (June 2016). "Design and Synthesis of Molecular Scaffolds with Anti-infective Activity". Tetrahedron. 72 (25): 3579–3592. doi:10.1016/j.tet.2016.01.044. PMC 4894353. PMID 27284210.
- ^ "Annual Report of the Chief Medical Officer - Infections and the rise of antimicrobial resistance" (PDF). UK NHS. 2011. Archived from the original (PDF) on 30 October 2013.
- ^ "Obama Administration Seeks To Ease Approvals For Antibiotics". NPR.org. NPR. 4 June 2013. Archived from the original on 13 March 2015. Retrieved 7 August 2016.
- ^ Prayle A, Watson A, Fortnum H, Smyth A (July 2010). "Side effects of aminoglycosides on the kidney, ear and balance in cystic fibrosis". Thorax. 65 (7): 654–658. doi:10.1136/thx.2009.131532. PMC 2921289. PMID 20627927.
- ^ Alene KA, Wangdi K, Colquhoun S, Chani K, Islam T, Rahevar K, et al. (September 2021). "Tuberculosis related disability: a systematic review and meta-analysis". BMC Medicine. 19 (1): 203. doi:10.1186/s12916-021-02063-9. PMC 8426113. PMID 34496845.
- ^ a b Walsh F (11 March 2013). "BBC News – Antibiotics resistance 'as big a risk as terrorism' – medical chief". BBC News. Bbc.co.uk. Archived from the original on 8 August 2018. Retrieved 12 March 2013.
- ^ a b Berida T, Adekunle Y, Dada-Adegbola H, Kdimy A, Roy S, Sarker S (May 2024). "Plant antibacterials: The challenges and opportunities". Heliyon. 10 (10): e31145. Bibcode:2024Heliy..1031145B. doi:10.1016/j.heliyon.2024.e31145. PMC 11128932. PMID 38803958.
- ^ Khor M (18 May 2014). "Why Are Antibiotics Becoming Useless All Over the World?". The Real News. Archived from the original on 18 May 2014. Retrieved 18 May 2014.
- ^ Nordrum A (2015). "Antibiotic Resistance: Why Aren't Drug Companies Developing New Medicines to Stop Superbugs?". International Business Times.
- ^ Gever J (4 February 2011). "Pfizer Moves May Dim Prospect for New Antibiotics". MedPage Today. Archived from the original on 14 December 2013. Retrieved 12 March 2013.
- ^ Schmitt S, Montalbán-López M, Peterhoff D, Deng J, Wagner R, Held M, et al. (May 2019). "Analysis of modular bioengineered antimicrobial lanthipeptides at nanoliter scale" (PDF). Nature Chemical Biology. 15 (5): 437–443. doi:10.1038/s41589-019-0250-5. PMID 30936500. S2CID 91188986. Archived (PDF) from the original on 18 April 2023. Retrieved 12 April 2023.
- ^ "Overview". Carb-X. Archived from the original on 28 March 2023. Retrieved 28 March 2023.
- ^ "Novel Gram Negative Antibiotic Now". CORDIS. doi:10.3030/853979. Archived from the original on 28 March 2023. Retrieved 28 March 2023.
- ^ "About". REPAIR Impact Fund. Archived from the original on 23 January 2019. Retrieved 28 March 2023.
- ^ "ADVANcing Clinical Evidence in Infectious Diseases (ADVANCE-ID)". sph.nus.edu.sg. Archived from the original on 28 March 2023. Retrieved 28 March 2023.
- ^ "About GARDP". GARDP. 27 September 2022. Archived from the original on 28 March 2023. Retrieved 28 March 2023.
- ^ Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, et al. (October 2021). "The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin". Drugs. 81 (15): 1703–1729. doi:10.1007/s40265-021-01611-0. PMC 8501344. PMID 34626339.
- ^ "Antibiotic resistance outwitted by supercomputers". University of Portsmouth. Archived from the original on 13 December 2021. Retrieved 13 December 2021.
- ^ König G, Sokkar P, Pryk N, Heinrich S, Möller D, Cimicata G, et al. (November 2021). "Rational prioritization strategy allows the design of macrolide derivatives that overcome antibiotic resistance". Proceedings of the National Academy of Sciences of the United States of America. 118 (46): e2113632118. Bibcode:2021PNAS..11813632K. doi:10.1073/pnas.2113632118. PMC 8609559. PMID 34750269.
- ^ Leppäranta O, Vaahtio M, Peltola T, Zhang D, Hupa L, Hupa M, et al. (February 2008). "Antibacterial effect of bioactive glasses on clinically important anaerobic bacteria in vitro". Journal of Materials Science. Materials in Medicine. 19 (2): 547–51. doi:10.1007/s10856-007-3018-5. PMID 17619981. S2CID 21444777.
- ^ Munukka E, Leppäranta O, Korkeamäki M, Vaahtio M, Peltola T, Zhang D, et al. (January 2008). "Bactericidal effects of bioactive glasses on clinically important aerobic bacteria". Journal of Materials Science. Materials in Medicine. 19 (1): 27–32. doi:10.1007/s10856-007-3143-1. PMID 17569007. S2CID 39643380.
- ^ Drago L, De Vecchi E, Bortolin M, Toscano M, Mattina R, Romanò CL (August 2015). "Antimicrobial activity and resistance selection of different bioglass S53P4 formulations against multidrug resistant strains". Future Microbiology. 10 (8): 1293–9. doi:10.2217/FMB.15.57. PMID 26228640.
- ^ Ermini ML, Voliani V (April 2021). "Antimicrobial Nano-Agents: The Copper Age". ACS Nano. 15 (4): 6008–6029. doi:10.1021/acsnano.0c10756. PMC 8155324. PMID 33792292.
- ^ Connelly E (18 April 2017). "Medieval medical books could hold the recipe for new antibiotics". The Conversation. Archived from the original on 25 December 2022. Retrieved 20 December 2019.
- ^ "AncientBiotics – a medieval remedy for modern day superbugs?" (Press release). University of Nottingham. 30 March 2015. Archived from the original on 21 October 2022. Retrieved 13 August 2019.
- ^ a b Kim DW, Cha CJ (March 2021). "Antibiotic resistome from the One-Health perspective: understanding and controlling antimicrobial resistance transmission". Experimental & Molecular Medicine. 53 (3): 301–309. doi:10.1038/s12276-021-00569-z. PMC 8080597. PMID 33642573.
- ^ Alcock BP, Huynh W, Chalil R, Smith KW, Raphenya AR, Wlodarski MA, et al. (January 2023). "CARD 2023: expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database". Nucleic Acids Research. 51 (D1): D690–D699. doi:10.1093/nar/gkac920. PMC 9825576. PMID 36263822.
- ^ "The Comprehensive Antibiotic Resistance Database". card.mcmaster.ca. Archived from the original on 28 March 2023. Retrieved 28 March 2023.
- ^ Florensa AF, Kaas RS, Clausen PT, Aytan-Aktug D, Aarestrup FM (January 2022). "ResFinder – an open online resource for identification of antimicrobial resistance genes in next-generation sequencing data and prediction of phenotypes from genotypes". Microbial Genomics. 8 (1). doi:10.1099/mgen.0.000748. PMC 8914360. PMID 35072601.
- ^ "ResFinder 4.1". cge.food.dtu.dk. Archived from the original on 28 March 2023. Retrieved 28 March 2023.
- ^ "Silent Killers: Fantastic Phages?". CBS News. 19 September 2002. Archived from the original on 10 February 2013. Retrieved 14 November 2017.
- ^ McAuliffe O, Ross RP, Fitzgerald GF (2007). "The New Phage Biology: From Genomics to Applications" (introduction)". In McGrath S, van Sinderen D (eds.). Bacteriophage: Genetics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-14-1. Archived from the original on 26 November 2020. Retrieved 11 August 2021.
- ^ Lin DM, Koskella B, Lin HC (August 2017). "Phage therapy: An alternative to antibiotics in the age of multi-drug resistance". World Journal of Gastrointestinal Pharmacology and Therapeutics. 8 (3): 162–173. doi:10.4292/wjgpt.v8.i3.162. PMC 5547374. PMID 28828194.
- ^ a b Salmond GP, Fineran PC (December 2015). "A century of the phage: past, present and future". Nature Reviews. Microbiology. 13 (12): 777–86. doi:10.1038/nrmicro3564. PMID 26548913. S2CID 8635034.
- ^ Letarov AV, Golomidova AK, Tarasyan KK (April 2010). "Ecological basis for rational phage therapy". Acta Naturae. 2 (1): 60–72. doi:10.32607/20758251-2010-2-1-60-71. PMC 3347537. PMID 22649629.
- ^ Parfitt T (June 2005). "Georgia: an unlikely stronghold for bacteriophage therapy". Lancet. 365 (9478): 2166–7. doi:10.1016/S0140-6736(05)66759-1. PMID 15986542. S2CID 28089251.
- ^ Golkar Z, Bagasra O, Pace DG (February 2014). "Bacteriophage therapy: a potential solution for the antibiotic resistance crisis". Journal of Infection in Developing Countries. 8 (2): 129–36. doi:10.3855/jidc.3573. PMID 24518621.
- ^ McCallin S, Alam Sarker S, Barretto C, Sultana S, Berger B, Huq S, et al. (September 2013). "Safety analysis of a Russian phage cocktail: from metagenomic analysis to oral application in healthy human subjects". Virology. 443 (2): 187–96. doi:10.1016/j.virol.2013.05.022. PMID 23755967.
- ^ Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM (March 2011). "Phage treatment of human infections". Bacteriophage. 1 (2): 66–85. doi:10.4161/bact.1.2.15845. PMC 3278644. PMID 22334863.
- ^ Onsea J, Soentjens P, Djebara S, Merabishvili M, Depypere M, Spriet I, et al. (September 2019). "Bacteriophage Application for Difficult-to-treat Musculoskeletal Infections: Development of a Standardized Multidisciplinary Treatment Protocol". Viruses. 11 (10): 891. doi:10.3390/v11100891. PMC 6832313. PMID 31548497.
Books
[edit]- Caldwell R, Lindberg D, eds. (2011). "Understanding Evolution" [Mutations are random]. University of California Museum of Paleontology. Archived from the original on 8 February 2012. Retrieved 15 August 2011.
- Reynolds LA, Tansey EM, eds. (2008). Superbugs and superdrugs : a history of MRSA : the transcript of a Witness Seminar held by the Wellcome Trust Centre for the History of Medicine at UCL, London, on 11 July 2006. London: Wellcome Trust Centre for the History of Medicine at UCL. ISBN 978-0-85484-114-1.
- Stemming the Superbug Tide: Just A Few Dollars More. OECD Health Policy Studies. Paris: OECD Publishing. 2018. doi:10.1787/9789264307599-en. ISBN 978-92-64-30758-2. S2CID 239804815.
Journals
[edit]- Arias CA, Murray BE (January 2009). "Antibiotic-resistant bugs in the 21st century – a clinical super-challenge". The New England Journal of Medicine. 360 (5): 439–443. doi:10.1056/NEJMp0804651. PMID 19179312. S2CID 205104375.
- "Special Issue: Ethics and Antimicrobial Resistance". Bioethics. 365 (33). 2019. Archived from the original on 9 March 2022. Retrieved 22 January 2020.
- Goossens H, Ferech M, Vander Stichele R, Elseviers M (2005). "Outpatient antibiotic use in Europe and association with resistance: a cross-national database study". Lancet. Group Esac Project. 365 (9459). Esac Project: 579–587. doi:10.1016/S0140-6736(05)17907-0. PMID 15708101. S2CID 23782228.
- Hawkey PM, Jones AM (September 2009). "The changing epidemiology of resistance" (PDF). The Journal of Antimicrobial Chemotherapy. 64 (Suppl 1): i3–10. doi:10.1093/jac/dkp256. PMID 19675017. Archived from the original on 17 February 2024. Retrieved 20 April 2018.
- Soulsby EJ (November 2005). "Resistance to antimicrobials in humans and animals". BMJ. 331 (7527): 1219–1220. doi:10.1136/bmj.331.7527.1219. PMC 1289307. PMID 16308360.
- "Alternatives to Antibiotics Reduce Animal Disease". Commonwealth Scientific and Industrial Research Organization. 9 January 2006. Archived from the original on 5 June 2011. Retrieved 26 April 2009.
- Cooke P, Rees-Roberts D (2017). CATCH. Archived from the original on 9 March 2022. Retrieved 23 February 2017. 16-minute film about a post-antibiotic world. Review: Sansom C (March 2017). "Media Watch: An intimate family story in a world without antibiotics". Lancet Infect Dis. 17 (3): 274. doi:10.1016/S1473-3099(17)30067-1.
Further reading
[edit]- Bancroft, EA (October 2007). "Antimicrobial resistance: it's not just for hospitals". JAMA. 298 (15): 1803–1804. doi:10.1001/jama.298.15.1803. PMC 2536104. PMID 17940239.
- Larson, E (2007). "Community factors in the development of antibiotic resistance". Annual Review of Public Health. 28 (1): 435–447. doi:10.1146/annurev.publhealth.28.021406.144020. PMID 17094768.
External links
[edit] Quotations related to Antimicrobial resistance at Wikiquote
Quotations related to Antimicrobial resistance at Wikiquote- WHO fact sheet on antimicrobial resistance
- Animation of Antibiotic Resistance Archived 28 September 2022 at the Wayback Machine
- Bracing for Superbugs: Strengthening environmental action in the One Health response to antimicrobial resistance UNEP, 2023.
- CDC Guideline "Management of Multidrug-Resistant Organisms in Healthcare Settings, 2006"
