Gallium scan
| Gallium-67 scan | |
|---|---|
| Synonyms | Gallium imaging |
| ICD-10-PCS | C?1?LZZ (planar) C?2?LZZ (tomographic) |
| ICD-9-CM | 92.18 |
| OPS-301 code | 3-70c |
| MedlinePlus | 003450 |
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active.[1] Both 67Ga and 68Ga salts have similar uptake mechanisms.[2] Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium-67 is imaged by a gamma camera, while the positron emission of gallium-68 is imaged by positron emission tomography (PET).
Gallium salts are taken up by tumors, inflammation, and both acute and chronic infection,[3][4] allowing these pathological processes to be imaged. Gallium is particularly useful in imaging osteomyelitis that involves the spine, and in imaging older and chronic infections that may be the cause of a fever of unknown origin.[5][6]
Gallium-68 DOTA scans are increasingly replacing octreotide scans (a type of indium-111 scan using octreotide as a somatostatin receptor ligand). The gallium-68 is bound to an octreotide derivative chemical such as DOTATOC and the positrons it emits are imaged by PET-CT scan. Such scans are useful in locating neuroendocrine tumors and pancreatic cancer.[7][8]
Gallium citrate scan
[edit]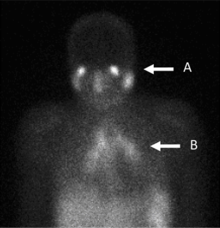
In the past, the gallium scan was the gold standard for lymphoma staging, until it was replaced by positron emission tomography (PET) using fludeoxyglucose (FDG).[9][10] Gallium imaging is still used to image inflammation and chronic infections, and it still sometimes locates unsuspected tumors as it is taken up by many kinds of cancer cells in amounts that exceed those of normal tissues. Thus, an increased uptake of gallium-67 may indicate a new or old infection, an inflammatory focus from any cause, or a cancerous tumor.
It has been suggested that gallium imaging may become an obsolete technique, with indium leukocyte imaging and technetium antigranulocyte antibodies replacing it as a detection mechanism for infections. For detection of tumors, especially lymphomas, gallium imaging is still in use, but may be replaced by fludeoxyglucose PET imaging in the future.[11]
In infections, the gallium scan has an advantage over indium leukocyte imaging in imaging osteomyelitis (bone infection) of the spine, lung infections and inflammation, and for chronic infections. In part this is because gallium binds to neutrophil membranes, even after neutrophil death. Indium leukocyte imaging is better for acute infections (where neutrophils are still rapidly and actively localizing to the infection), and also for osteomyelitis that does not involve the spine, and for abdominal and pelvic infections. Both the gallium scan and indium leukocyte imaging may be used to image fever of unknown origin (elevated temperature without an explanation). However, the indium leukocyte scan will image only the 25% of such cases which are caused by acute infections, while gallium will also localize to other sources of fever, such as chronic infections and tumors.[12][13]
Mechanism
[edit]The body generally handles Ga3+ as though it were ferric iron (Fe-III), and thus the free isotope ion is bound (and concentrates) in areas of inflammation, such as an infection site, and also areas of rapid cell division.[14] Gallium (III) (Ga3+) binds to transferrin, leukocyte lactoferrin, bacterial siderophores, inflammatory proteins, and cell-membranes in neutrophils, both living and dead.[15]
Lactoferrin is contained within leukocytes. Gallium may bind to lactoferrin and be transported to sites of inflammation, or binds to lactoferrin released during bacterial phagocytosis at infection sites (and remains due to binding with macrophage receptors).[16] Gallium-67 also attaches to the siderophore molecules of bacteria themselves, and for this reason can be used in leukopenic patients with bacterial infection (here it attaches directly to bacterial proteins, and leukocytes are not needed).[17] Uptake is thought to be associated with a range of tumour properties including transferring receptors, anaerobic tumor metabolism and tumor perfusion and vascular permeability.[18][19]
Common indications
[edit]- Whole-body survey to localize source of fever in patients with fever of unknown origin.[20]
- Detection of pulmonary and mediastinal inflammation/infection, especially in the immunocompromised patient.[21]
- Evaluation and follow-up of active lymphocytic or granulomatous inflammatory processes such as sarcoidosis or tuberculosis.[22]
- Diagnosing vertebral osteomyelitis and/or disk space infection where gallium-67 is preferred over labeled leukocytes.
- Diagnosis and follow-up of medical treatment of retroperitoneal fibrosis.
- Evaluation and follow-up of drug-induced pulmonary toxicity (e.g. Bleomycin, Amiodarone)
- Evaluation of patients who are not candidates for WBC scans (WBC count less than 6,000).
Note that all of these conditions are also seen in PET scans using the gallium-68.
Technique
[edit]The main (67Ga) technique uses scintigraphy to produce two-dimensional images. After the tracer has been injected, images are typically taken by a gamma camera at 24, 48, and in some cases, 72, and 96 hours later.[23][24] Each set of images takes 30–60 minutes, depending on the size of the area being imaged. The resulting image will have bright areas that collected large amounts of tracer, because inflammation is present or rapid cell division is occurring. Single-photon emission computed tomography (SPECT) images may also be acquired. In some imaging centers, SPECT images may be combined with computed tomography (CT) scan using either fusion software or SPECT/CT hybrid cameras to superimpose both physiological image-information from the gallium scan, and anatomical information from the CT scan.
A common injection dose is around 150 megabecquerels.[25] Imaging should not usually be sooner than 24 hours as high background at this time produces false negatives. Forty-eight-hour whole body images are appropriate. Delayed imaging can be obtained even 1 week or longer after injection if bowel is confounding. SPECT can be performed as needed. Oral laxatives or enemas can be given before imaging to reduce bowel activity and reduce dose to large bowel; however, the usefulness of bowel preparation is controversial.[24]
10% to 25% of the dose of gallium-67 is excreted within 24 hours after injection (the majority of which is excreted through the kidneys). After 24 hours the principal excretory pathway is colon.[24] The "target organ" (organ that receives the largest radiation dose in the average scan) is the colon (large bowel).[23]
In a normal scan, uptake of gallium is seen in wide range of locations which do not indicate a positive finding. These typically include soft tissues, liver, and bone. Other sites of localisation can be nasopharyngeal and lacrimal glands, breasts (particularly in lactation or pregnancy), normally healing wounds, kidneys, bladder and colon.[26]
Gallium PSMA scan
[edit]

The positron emitting isotope, 68Ga, can be used to target prostate-specific membrane antigen (PSMA), a protein which is present in prostate cancer cells. The technique has been shown to improve detection of metastatic disease compared to MRI or CT scans.[27]
In December 2020, the U.S. Food and Drug Administration (FDA) approved 68Ga PSMA-11 for medical use in the United States.[28][29] It is indicated for positron emission tomography (PET) of prostate specific membrane antigen (PSMA) positive lesions in men with prostate cancer.[30][29] It is manufactured by the UCLA Biomedical Cyclotron Facility.[29] The FDA approved 68Ga PSMA-11 based on evidence from two clinical trials (Trial 1/NCT0336847 identical to NCT02919111 and Trial 2/NCT02940262 identical to NCT02918357) of male participants with prostate cancer.[29] Some participants were recently diagnosed with the prostate cancer.[29] Other participants were treated before, but there was suspicion that the cancer was spreading because of rising prostate specific antigen or PSA.[29] The trials were conducted at two sites in the United States.[29]
The FDA considers 68Ga PSMA-11 to be a first-in-class medication.[31]
Common indications
[edit]Gallium PSMA scanning is recommended primarily in cases of biochemical recurrence of prostate cancer, particularly for patients with low PSA values, and in patients with high risk disease where metastases are considered likely.[32][33]
Technique
[edit]An intravenous administration of 1.8–2.2 megabecquerels of 68Ga PSMA-11 per kilogram of bodyweight is recommended. Imaging should commence approximately 60 minutes after administration with an acquisition from mid-thigh to the base of the skull.[32][34]
Gallium DOTA scans
[edit]68Ga DOTA conjugated peptides (including 68Ga DOTA-TATE, DOTA-TOC and DOTA-NOC) are used in positron emission tomography (PET) imaging of neuroendocrine tumours (NETs). The scan is similar to the SPECT octreotide scan in that an octreotide-based somatostatin analogue (such as edotreotide) is used as the radioligand, and there are similar indications and uses as ocreotide scans, however image quality is significantly improved.[35] Somatostatin receptors are overexpressed in many NETs, so that the 68Ga DOTA conjugated peptide is preferentially taken up in these locations, and visualised on the scan.[36] As well as diagnosis and staging of NETs, 68Ga DOTA conjugated peptide imaging may be used for planning and dosimetry in preparation for lutetium-177 or yttrium-90 DOTA therapy.[37][38]
In June 2016, Netspot (kit for the preparation of gallium Ga-68 dotatate injection) was approved for medical use in the United States.[39][40]
In August 2019, 68Ga edotreotide injection (68Ga DOTATOC) was approved for medical use in the United States for use with PET imaging for the localization of somatostatin receptor positive neuroendocrine tumors (NETs) in adults and children.[41][42][43]
The U.S. Food and Drug Administration (FDA) approved 68Ga edotreotide (DOTATOC) based on evidence from three clinical trials (Trial 1/NCT#1619865, Trial 2/NCT#1869725, Trial 3/NCT#2441062) of 334 known or suspected neuro-endocrine tumors.[42] The trials were conducted in the United States.[42]
Gallium (68Ga) oxodotreotide was approved for medical use in Canada as Netspot in July 2019,[44] and as Netvision in May 2022.[45]
The combination germanium (68Ge) chloride / gallium (68Ga) chloride was approved for medical use in the European Union in August 2024.[46]
Radiochemistry of gallium-67
[edit]Gallium-67 citrate is produced by a cyclotron. Charged particle bombardment of enriched Zn-68 is used to produce gallium-67. The gallium-67 is then complexed with citric acid to form gallium citrate. The half-life of gallium-67 is 78 hours.[47] It decays by electron capture, then emits de-excitation gamma rays that are detected by a gamma camera. Primary emission is at 93 keV (39% abundance), followed by 185 keV (21%) and 300 keV (17%).[48]: 64 For imaging, multiple gamma camera energy windows are used, typically centred around 93 and 184 keV or 93, 184, and 296 keV.[24]
Radiochemistry of gallium-68
[edit]Gallium-68, which has a 68 minutes half-life, is produced in a gallium-68 generator by decay of germanium-68 with a 271 day half-life or by the irradiation of zinc-68 through a low energy cyclotron. Use of a generator means a supply of 68Ga can be produced easily with minimal infrastructure, for example at sites without a cyclotron, commonly used to produce other PET isotopes. It decays by positron emission and electron capture into zinc-68.[49] Maximum energy of positron emission is at 1.9 MeV.[48]: 65
References
[edit]- ^ Treves ST (2014). Pediatric nuclear medicine and molecular imaging (4th ed.). Springer. p. 480. ISBN 9781461495512.
- ^ Jain SK (2017). Imaging Infections: From Bench to Bedside. Springer. p. 34. ISBN 9783319545929. Archived from the original on 21 February 2022. Retrieved 23 June 2017.
- ^ Verberne SJ and O. P. P. Temmerman (2017). 12 - Imaging of prosthetic joint infections Archived 21 February 2022 at the Wayback Machine - Arts, J.J. Chris. Management of Periprosthetic Joint Infections (PJIs). J. Geurts, Woodhead Publishing: 259-285.
- ^ Verberne SJ, Raijmakers PG, Temmerman O (2016). "The Accuracy of Imaging Techniques in the Assessment of Periprosthetic Hip Infection: A Systematic Review and Meta-Analysis". The Journal of Bone and Joint Surgery. American Volume. 98 (19): 1638–1645. doi:10.2106/jbjs.15.00898. PMID 27707850. S2CID 9202184. Archived from the original on 16 December 2016. Retrieved 18 December 2016.
- ^ Termaat MF, Raijmakers PG, Scholten HJ, Bakker FC, Patka P, Haarman HJ (November 2005). "The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis". The Journal of Bone and Joint Surgery. American Volume. 87 (11): 2464–71. doi:10.2106/JBJS.D.02691 (inactive 11 November 2024). PMID 16264122. S2CID 26280068.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Becker W (October 1995). "The contribution of nuclear medicine to the patient with infection". European Journal of Nuclear Medicine. 22 (10): 1195–1211. doi:10.1007/BF00800606. PMID 8542906. S2CID 19293222.
- ^ Hofman M, Kong G, Neels O, Eu P, Hong E, Hicks R (2012). "High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours". Journal of Medical Imaging and Radiation Oncology. 56 (1): 40–47. doi:10.1111/j.1754-9485.2011.02327.x. PMID 22339744. S2CID 21843609.
- ^ Scott, A, Howe J (2018). "Management of Small Bowel Neuroendocrine Tumors". Journal of Oncology Practice. 14 (8): 471–482. doi:10.1200/JOP.18.00135. PMC 6091496. PMID 30096273.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Bryan RN (2010). Introduction to the science of medical imaging. Cambridge: Cambridge University Press. p. 200. ISBN 9780521747622. Archived from the original on 21 February 2017. Retrieved 20 February 2017.
- ^ Bleeker-Rovers CP, Vos FJ, van der Graaf WT, Oyen WJ (16 June 2011). "Nuclear Medicine Imaging of Infection in Cancer Patients (With Emphasis on FDG-PET)". The Oncologist. 16 (7): 980–991. doi:10.1634/theoncologist.2010-0421. PMC 3228133. PMID 21680576.
- ^ Ziessman HA, O'Malley JP, Thrall JH (2013). Nuclear Medicine: The Requisites E-Book. Elsevier Health Sciences. p. 281. ISBN 978-0323112925.
- ^ Palestro CJ (April 1994). "The current role of gallium imaging in infection". Seminars in Nuclear Medicine. 24 (2): 128–141. doi:10.1016/S0001-2998(05)80227-2. PMID 8023169.
- ^ Shields TW, LoCicero J, Reed CE, Feins RH (2009). General Thoracic Surgery. Lippincott Williams & Wilkins. p. 2106. ISBN 9780781779821.
- ^ Love C, Palestro CJ (June 2004). "Radionuclide imaging of infection". Journal of Nuclear Medicine Technology. 32 (2): 47–57, quiz 58–9. PMID 15175400. Archived from the original on 7 November 2016. Retrieved 20 February 2017.
- ^ Tsan MF (January 1985). "Mechanism of gallium-67 accumulation in inflammatory lesions". Journal of Nuclear Medicine. 26 (1): 88–92. PMID 3880816.
- ^ Greenberg AM, Prein J (2007). Craniomaxillofacial reconstructive and corrective bone surgery principles of internal fixation using AO/ASIF technique. New York: Springer. p. 79. ISBN 9780387224275.
- ^ Weiner R (1996). "The mechanism of 67Ga localization in malignant disease". Nuclear Medicine and Biology. 23 (6): 745–751. doi:10.1016/0969-8051(96)00119-9. PMID 8940716.
- ^ Biersack HJ, Freeman LM (2007). Clinical nuclear medicine. Berlin: Springer. p. 324. ISBN 978-3-540-28026-2.
- ^ Hoffer P (1980). "Gallium: mechanisms". Journal of Nuclear Medicine. 21 (3): 282–5. PMID 6988551. Archived from the original on 7 July 2022. Retrieved 20 February 2017.
- ^ "Gallium scan". MedlinePlus. Archived from the original on 14 September 2017. Retrieved 14 September 2017.
- ^ "ACR–SPR Practice Parameter for the Performance of Scintigraphy for Inflammation and Infection" (PDF). American College of Radiology. 2014. Archived from the original (PDF) on 16 October 2015. Retrieved 14 September 2017.
- ^ "Lung gallium scan". MedlinePlus. Archived from the original on 14 September 2017. Retrieved 14 September 2017.
- ^ a b Bombardieri E, Aktolun C, Baum RP, Bishof-Delaloye A, Buscombe J, Chatal JF, et al. (2 September 2003). "67Ga Scintigraphy Procedure Guidelines for Tumour Imaging" (PDF). EANM. Archived (PDF) from the original on 26 October 2020. Retrieved 14 September 2017.
- ^ a b c d "Society of Nuclear Medicine Procedure Guideline for Gallium Scintigraphy in Inflammation" (PDF). SNMMI. 2 June 2004. Archived (PDF) from the original on 21 October 2016. Retrieved 7 September 2016.
- ^ "Notes for Guidance on the Clinical Administration of Radiopharmaceuticals and Use of Sealed Radioactive Sources" (PDF). Administration of Radioactive Substances Advisory Committee. January 2016. Archived (PDF) from the original on 24 September 2016. Retrieved 7 September 2016.
- ^ Palestro CJ (2012). "SPECT and PET in the Assessment of Bone Infections". In Fogelman I, Gnanasegaran G, van der Wall H (eds.). Radionuclide and hybrid bone imaging. Berlin: Springer. pp. 523–559. doi:10.1007/978-3-642-02400-9_20. ISBN 978-3-642-02399-6.
- ^ Maurer T, Eiber M, Schwaiger M, Gschwend JE (23 February 2016). "Current use of PSMA–PET in prostate cancer management". Nature Reviews Urology. 13 (4): 226–235. doi:10.1038/nrurol.2016.26. PMID 26902337. S2CID 2448922.
- ^ "Drug Approval Package: Gallium Ga 68 PSMA-11". U.S. Food and Drug Administration (FDA). 16 December 2020. Archived from the original on 26 January 2021. Retrieved 25 December 2020.
- ^ a b c d e f g "Drug Trials Snapshot: Ga 68 PSMA-11". U.S. Food and Drug Administration (FDA). 1 December 2020. Archived from the original on 10 December 2020. Retrieved 11 December 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "GALLIUM GA 68 PSMA-11 Labeling-Package Insert" (PDF). Drugs@FDA. University of California, Los Angeles. 17 November 2021. Archived (PDF) from the original on 13 May 2022. Retrieved 18 March 2022.
- ^ "New Drug Therapy Approvals 2020". U.S. Food and Drug Administration (FDA). 31 December 2020. Archived from the original on 18 January 2021. Retrieved 17 January 2021.
- ^ a b Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. (10 March 2017). "68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0". European Journal of Nuclear Medicine and Molecular Imaging. 44 (6): 1014–1024. doi:10.1007/s00259-017-3670-z. PMID 28283702. S2CID 5882407.
- ^ Rai BP, Baum RP, Patel A, Hughes R, Alonzi R, Lane T, et al. (September 2016). "The Role of Positron Emission Tomography With 68Gallium (Ga)-Labeled Prostate-specific Membrane Antigen (PSMA) in the Management of Patients With Organ-confined and Locally Advanced Prostate Cancer Prior to Radical Treatment and After Radical Prostatectomy". Urology. 95: 11–15. doi:10.1016/j.urology.2015.12.048. PMID 26790588.
- ^ Afaq A, Batura D, Bomanji J (14 February 2017). "New frontiers in prostate cancer imaging: clinical utility of prostate-specific membrane antigen positron emission tomography". International Urology and Nephrology. 49 (5): 803–810. doi:10.1007/s11255-017-1541-y. PMID 28197764. S2CID 3902900.
- ^ Mojtahedi A, Thamake S, Tworowska I, Ranganathan D, Delpassand ES (15 August 2014). "The value of 68Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature". American Journal of Nuclear Medicine and Molecular Imaging. 4 (5): 426–434. ISSN 2160-8407. PMC 4138137. PMID 25143861.
- ^ Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, et al. (2 July 2010). "Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE" (PDF). European Journal of Nuclear Medicine and Molecular Imaging. 37 (10): 2004–2010. doi:10.1007/s00259-010-1512-3. PMID 20596866. S2CID 11469889. Archived (PDF) from the original on 17 May 2017. Retrieved 6 October 2017.
- ^ Kam BL, Teunissen JJ, Krenning EP, de Herder WW, Khan S, van Vliet EI, et al. (3 March 2012). "Lutetium-labelled peptides for therapy of neuroendocrine tumours". European Journal of Nuclear Medicine and Molecular Imaging. 39 (S1): 103–112. doi:10.1007/s00259-011-2039-y. PMC 3304065. PMID 22388631.
- ^ Taïeb D, Garrigue P, Bardiès M, Abdullah AE, Pacak K (October 2015). "Application and Dosimetric Requirements for Gallium-68–labeled Somatostatin Analogues in Targeted Radionuclide Therapy for Gastroenteropancreatic Neuroendocrine Tumors". PET Clinics. 10 (4): 477–486. doi:10.1016/j.cpet.2015.06.001. PMC 4617555. PMID 26384594.
- ^ "Netspot (kit for the preparation of gallium Ga 68 dotatate injection)". U.S. Food and Drug Administration (FDA). 21 June 2016. Archived from the original on 31 March 2021. Retrieved 18 October 2020.
- Lay summary in: "Summary Review: Application number: 208547Orig1s000" (PDF). Center for Drug Evaluation and Research. 30 May 2016.
- ^ "Netspot- 68ga-dotatate kit". DailyMed. 23 October 2019. Archived from the original on 22 September 2020. Retrieved 18 October 2020.
- ^ "GA-68-DOTATOC- edotreotide gallium ga-68 injection, solution". DailyMed. 3 September 2019. Archived from the original on 7 July 2022. Retrieved 17 March 2020.
- ^ a b c "Drug Trials Snapshots: Ga-68-DOTATOC". U.S. Food and Drug Administration (FDA). 21 August 2019. Archived from the original on 13 December 2019. Retrieved 17 March 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Drug Approval Package: Gallium Dotatoc GA 68". U.S. Food and Drug Administration (FDA). 23 September 2019. Archived from the original on 6 April 2021. Retrieved 18 October 2020.
- ^ "Summary Basis of Decision (SBD) for Netspot". Health Canada. 23 October 2014. Archived from the original on 31 May 2022. Retrieved 29 May 2022.
- ^ "Summary Basis of Decision - NETVision". Health Canada. 26 May 2022. Archived from the original on 7 July 2022. Retrieved 7 July 2022.
- ^ "GalliaPharm EPAR". European Medicines Agency (EMA). 1 August 2024. Retrieved 26 August 2024.
- ^ IAEA (2009). Cyclotron produced radionuclides: physical characteristics and production methods (PDF). Vienna: International Atomic Energy Agency. p. 116. ISBN 9789201069085. Archived (PDF) from the original on 9 March 2017. Retrieved 7 September 2016.
- ^ a b Delacroix D, Guerre JP, Leblanc P, Hickman C (2002). Radionuclide and Radiation Protection Data Handbook (2nd ed.). Ashford: Nuclear Technology Publishing. ISBN 978-1870965873.
- ^ Bé MM, Chisté V, Mougeot X, Chechev V, Kondev F, Nichols AL, et al. (2013). Monographie BIPM: Table of radionuclides Vol. 7. Paris: Bureau International des Poids et Mesures. p. 33. ISBN 9789282222485. Archived from the original on 23 August 2017. Retrieved 14 September 2017.
