Talk:Electromagnetic absorption by water
| This article is rated Start-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | ||||||||||||||||||
‹See TfM›
| ||||||||||||||||||
A fact from Electromagnetic absorption by water appeared on Wikipedia's Main Page in the Did you know column on 5 November 2007. The text of the entry was as follows:
|
microwaves?
[edit]The article's title makes it sound like it's talking about the whole electromagnetic spectrum, but in fact it only deals with visible and infrared. What about microwaves, for example, which are of considerable practical interest?--75.83.69.196 (talk) 17:54, 24 July 2011 (UTC)
- The intro now says Liquid water has no rotational spectrum but does absorb in the microwave region. I think it would be more accurate to say that liquid water has no discrete rotational spectrum, or that the microwave spectrum cannot be resolved into discrete rotational lines. A continuous spectrum is still a spectrum, and the microwave absorption is still due to rotational transitions.
- Also we could explain that the microwave lines are broadened and not resolved because the rotational states are short-lived in the presence of frequent collisions in the liquid phase. Dirac66 (talk) 15:16, 4 November 2012 (UTC)
- We need to be able to cite a source where the microwave spectrum of liquid water is explained. I found this http://www.lsbu.ac.uk/water/microwave.html] which talks of switching in the hydrogen-bond network. Petergans (talk) 15:28, 4 November 2012 (UTC)
- Ah, thank you for reminding me about H-bonds. Now I remember that the liquid water molecules do not rotate freely, which is why there are no rotational states and rotational spectrum. So perhaps the sentence I quoted above should be modified to "Liquid water has no rotational spectrum because the molecules are hydrogen-bonded and do not rotate freely. However there is absorption in the microwave region due to ... (switching in the hydrogen-bonded network?)". I would prefer that you write a definitive version rather than me, since you are clearly more familiar with the literature on this. Dirac66 (talk) 17:27, 4 November 2012 (UTC)
- Rotational fine structure is absent from the spectra of all liquids. I've added a reference in the Microwave section. Is this enough or do you think that the lead should have more detail? [Comment by Petergans]
- Here:
- http://www.springer.com/cda/content/document/cda_downloaddocument/9780387307534-c2.pdf
- is a table with rotational transitions in liquid water (page 46), at least one at 25 μm (and 20 μm in ice !) and librations being also rotations, but limited.Darekk2 (talk) 20:00, 4 November 2012 (UTC)
- Rotational fine structure is absent from the spectra of all liquids. I've added a reference in the Microwave section. Is this enough or do you think that the lead should have more detail? [Comment by Petergans]
- Ah, thank you for reminding me about H-bonds. Now I remember that the liquid water molecules do not rotate freely, which is why there are no rotational states and rotational spectrum. So perhaps the sentence I quoted above should be modified to "Liquid water has no rotational spectrum because the molecules are hydrogen-bonded and do not rotate freely. However there is absorption in the microwave region due to ... (switching in the hydrogen-bonded network?)". I would prefer that you write a definitive version rather than me, since you are clearly more familiar with the literature on this. Dirac66 (talk) 17:27, 4 November 2012 (UTC)
- We need to be able to cite a source where the microwave spectrum of liquid water is explained. I found this http://www.lsbu.ac.uk/water/microwave.html] which talks of switching in the hydrogen-bond network. Petergans (talk) 15:28, 4 November 2012 (UTC)
- I think the article should indicate (in the lead or elsewhere) what is the physical mechanism of microwave absorption by liquid water, rather than just saying there is no (free) rotational spectrum. Is it libration (oscillatory rotational motion) as Darekk2's reference indicates? My intuition says yes, but the Wiki article Libration (molecule) says that libration is seen in the IR region (with a reference which I have not yet read). Is it related to H-bond switching, perhaps an excitation of the O-H...O asymmetric stretch? But I would expect that to be in the IR also. So what is the right answer?
- Also it would be good to indicate the physical mechanism for the line broadening responsible for the absence of a discrete spectrum, for the specific case of water. Is it collisions? effect of H-bond switching? or of libration?
- As should be clear, this is outside my detailed knowledge so I have more questions than answers. But given the importance of microwave absorption by water in all of our kitchens (as Petergans' reference above points out), I think the questions are important.
Dirac66 (talk) 02:58, 5 November 2012 (UTC)
- This will take us into very deep water (sic!). The principle is quite straight forward. The H-bond network is a medium-scale structure somewhere between the structure of ice and a completely random structure. It has been the subject of serious molecular mechanics calculations. One interpretation is that there are small clusters, of various sizes, of water molecules linked by hydrogen bonds. These clusters are in dynamic equilibrium with each other on a picosecond time-scale. A complicating factor is that H-bond hopping can also occur (Grotthus mechanism). Hence, by a big conceptual jump, the broadness of the microwave absorption band. Some of it is in Water model, but not the effect of H-bonding on the width of absorption bands, which is a general phenomenon with a tough quantum mechanical explanation which I have seen, somewhere. I don't know where to look for citations to back all this up.
- On a personal note, this brings back memories of my student days. At my college the great J.D. Bernal (remember the Bernal-Fowler model?) had his technician, who was a personal friend, build a ball-and-stick model of the water structure. I saw the actual model; it was not far off what was later found using computers. Petergans (talk) 11:39, 5 November 2012 (UTC)
- OK, thanks. I think you have now answered the second of my two questions and briefly explained the origin of the width of the spectral bands. Of course this will not apply to non H-bonded liquids which must have other mechanisms, but I will accept your explanation as the dominant factor(s) for water which is the subject of this article.
- What about my first question - where does the energy of excitation go? Libration? Breaking of H-bonds? or other? Dirac66 (talk) 03:09, 6 November 2012 (UTC)
- The general answer is that absorbed light energy can be dissipated by "non-radiative transfer". In practice this means it is converted into kinetic energy, that is heat, via molecular collisions. Petergans (talk) 07:55, 6 November 2012 (UTC)
- Yes, I understand that, but my question concerns the first absorption step in the liquid state. In water vapour for comparison, the first step is rotational excitation which absorbs the microwave, followed by non-radiative transfer to heat which will be slow since there are few collisions. In liquid water, I would now assume that the first step is librational excitation which absorbs the microwave, followed by fast non-radiative transfer to heat in frequent collisions to heat, with or without hydrogen-bond switching. Is this correct? Dirac66 (talk) 12:31, 6 November 2012 (UTC)
- The general answer is that absorbed light energy can be dissipated by "non-radiative transfer". In practice this means it is converted into kinetic energy, that is heat, via molecular collisions. Petergans (talk) 07:55, 6 November 2012 (UTC)
Energy is absorbed when one cluster changes to another. This is a co-operative process with some H-bond(s) being broken and other H-bond(s) being formed. I don't see this as involving single molecules.
Good news. I have obtained permission from the copyright holder to show Fig. 1 from http://www.lsbu.ac.uk/water/microwave.html. I think it will be enough to show the spectra without going deeply into the theory. Petergans (talk) 16:49, 6 November 2012 (UTC)
Atmospheric effects - Problem with concept of thermal runaway
[edit]"In the atmospheric window between approximately 8000 and 14000 nm, in the far-infrared spectrum, carbon dioxide and water absorption is weak.[28] This window allows most of the thermal radiation in this band to be radiated out to space, keeping the Earth's atmosphere from going into thermal runaway. This band is also used for remote sensing of the Earth from space, for example with thermal Infrared imaging."
This suggests the only way the atmosphere cools is via the ground, which would mean the atmosphere just above the ground would be the coolest part. Instead there is a local cool zone of about -50 degrees Celsius at 12km altitude both day and night. Thus water vapour, liquid and/or ice is directly emitting net radiation into space at this altitude.
As you ascend from ground level the concentration of water reduces. Eventually water-specific radiation emissions heading upward encounter no more water molecules, and so escapes into space. The water takes the heat of neighboring greenhouse and non-greenhouse gases (eg. nitrogen, oxygen, argon and carbon dioxide) and emits this energy into space leaving a cold low pressure region. This enables convection and the water cycle. Richard. — Preceding unsigned comment added by 121.214.19.133 (talk) 10:45, 2 November 2013 (UTC)
The text asserts CO2 accounts for 26% "of the greenhouse effect." The reference [25] does not back up that assertion. In fact, 25 asserts CO2 is largely irrelevant compared to H2O. I cannot find any source that provides that 26% figure, nor a description of how to derive it. Frankly, given the overlap of CO2 with H2O, and that humidity is uniformly 100% over and near bodies of water, I can't see how the 26% figure can hold up. Paulsnx2 (talk) 18:06, 13 September 2022 (UTC)
Extensive revision
[edit]The article has been extensively revised. All comments welcome. Petergans (talk) 20:30, 2 November 2012 (UTC)
- It is good to make order, but diagrams showing entire water absorption spectrum including the UV region and section about absorption in the visible portion of electromagnetic radiation should be restored. Darekk2 (talk) 21:02, 2 November 2012 (UTC)
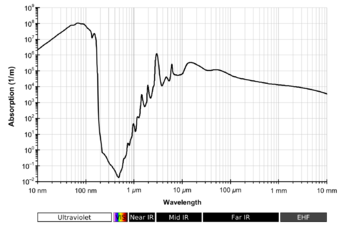
The diagram on the right looks wrong to me. Because of the logarithmic scale on the x-axis, resolution is ridiculously poor: O-H stretching and H-O-H bending are not even shown as separate absorption bands. In this sense it is inconsistent with the spectrum shown at the top of the article, so it's better left out. Also , the NIR region is shown as completerly featureless, which is nonsense - see near infrared spectroscopy.
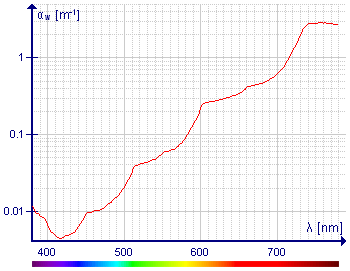
I don't know what to make of the diagram on the left. What is the point of showing the absorbance at path lengths up to 100 m? The average reader will look at this diagram and wonder why water used in everyday life appears to be colourless. The logarithmic scale on the y axis is very misleading. I have measured the absorbance of the band at ca. 700 nm with a Perkin-Elmer spectrophotometer and the baseline, looked pretty flat. I think this diagram is for specialists only, so I've restored the references but still left the diagram out.
BTW, I recently filled a new, white, bath tub with cold water. It was distintly blue, but this is, of course, due to Rayleigh scattering, not light absorption. I took out the picture of the divers because the blue colour is not due to absorption. Petergans (talk) 09:53, 3 November 2012 (UTC)
- This is article about absorption at all but not absorption in the infrared, so first diagram should be restored.
- Vertical axis of the second diagram is always logarithmical in the literature, moreover in other case that diagram would be unclear in the lower range of absorbance because differences are huge. Also peaks wouldn't be so distinguishable. This is very important graph because people deal with the water, i. e. taking underwater pictures. Visible light absorption is important for marine life too, like plants. So visible range is nedded.
- Pictures of divers. Color of water seen not from the above, but from underwater (like color of a white sheet seen in the water) is a result of not only absorption by water and not only absorption. This particular light is indirect, but it was filtered by the water. And light reflected directly from the reef or rock is not scaterred. Can you aprove citing literature, that absorption is not important in the water ? If you would be right, diagram showing absorption of the water in the visible range would be also result of the Rayleigh scattering, but it isn't. It shows even vibrational transitions. In such situation all absorption spectra of small particles would be result of Rayleigh scatering, not absorption. You are very wrong, this is nonsense.
- Why the water is blue
- Water absorption spectrum
- "Water is very slightly blue in color [131]c as overtone and combination vibrational absorption bands"
- Shedding new light on light in the ocean
- I have measured the absorbance of the band at ca. 700 nm with a Perkin-Elmer spectrophotometer and the baseline, looked pretty flat
- I have measured the absorbance thousands times before fashi hunt in Poland on me organized by bandits and thieves ruling Poland and USA reverting attention from themselves (followed by reducing of that madness by many people) and beginning of this dark death, but the light in the water is filtered by many meter water layer, not 1 cm. And in such situation even small absorption is important. In worst case that picture or text on the left of it needs additional explanation. Darekk2 (talk) 15:19, 3 November 2012 (UTC)
- Why the water is blue
External links modified
[edit]Hello fellow Wikipedians,
I have just modified 2 external links on Electromagnetic absorption by water. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added
{{dead link}}tag to http://144.206.159.178/FT/627/63662/1082676.pdf - Added archive https://web.archive.org/web/20071015144228/http://spt.uchicago.edu:80/public/southpole.html to http://spt.uchicago.edu/public/southpole.html
- Added archive https://web.archive.org/web/20110726224839/http://geography.swan.ac.uk/personal/prjn/papers/Prietoetal2005.pdf to http://geography.swan.ac.uk/personal/prjn/papers/Prietoetal2005.pdf
When you have finished reviewing my changes, please set the checked parameter below to true or failed to let others know (documentation at {{Sourcecheck}}).
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 07:11, 22 December 2016 (UTC)
External links modified (January 2018)
[edit]Hello fellow Wikipedians,
I have just modified one external link on Electromagnetic absorption by water. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://archive.is/20130416222417/http://spectra.iao.ru/1024x563/en/mol/survey/1/ to http://spectra.iao.ru/1024x563/en/mol/survey/1/
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 00:05, 26 January 2018 (UTC)
Easy red-green accessibility request
[edit]I'm colorblind (I have a color vision deficit, deuteranomaly), and although I can usually see the difference between red and green, or red and brown, I cannot actually see on the first graphic currently. Although it is called "red-green colorblindness", this pair is usually a small problem. Green and brown are nearly impossible for me, though.
I am asking for help with the first graphic, which is absorption (vertical axis) versus wavelength (horizontal axis), and it has red, green, and blue lines (apparently).
Could someone with intact color vision please amend the description of the first graphic to say "(red, top)" and "(green, bottom)" or, much less likely, "(red, bottom)" and "(green, top)", depending on which pair is correct? I cannot tell visually which pair is correct. My knowledge of chemistry and physics leads me to suspect "(red, top)" is correct, because the top line has higher absorption almost everywhere, which I suspect is consistent with liquid water, which is higher density. I'm also guessing that the hydrogen bonding in liquid and solid phases may tend to smooth out the spectral peaks, but I'm not sure if this effect is significant.
I'm not requesting this here, but: It would help if there was a color legend or key, with a swatch of the color, because then I could visually match them. The top line is brighter than the bottom line, for me. At first I was thinking the top line was "frog green" (bright) and the bottom was "blood red" (medium-dark), but then I realized that doesn't fit the science as well. Green meaning "go" also tends to be bright. I now suspect the top is "candy apple red" (bright) and the bottom is dark green. Let this be a lesson that color legends with swatches are helpful, because I can perceive brightness when the hue (and name) don't help. It's still a challenge, especially if the lines are very thin.
I know that "top" and "bottom" won't work for other graphs in general, but they should work here, and I hope some well-intentioned person doesn't delete them as redundant information. Thank you. Fluoborate (talk) 13:09, 29 July 2024 (UTC)
Clarity of Writing
[edit]This article needs to be overhauled entirely. The scientific information is presented in such a way that it is not accessible to the general public. It seems as if companies manufacturing UV caps for purifying water rely on the wordiness of this article to confuse people into spending way too much money on a UV light which would not "photodissociate water into radicals that can purify/cure disease". 129.3.116.139 (talk) 17:16, 15 November 2024 (UTC)


