TREM1
| TREM1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
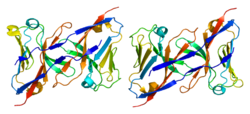 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | TREM1, CD354, TREM-1, triggering receptor expressed on myeloid cells 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 605085; MGI: 1930005; HomoloGene: 10243; GeneCards: TREM1; OMA:TREM1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Triggering receptor expressed on myeloid cells 1 (TREM1) is an immunoglobulin (Ig) superfamily transmembrane protein that, in humans, is encoded by the TREM1 gene.[5][6][7] TREM1 is constitutively expressed on the surface of peripheral blood monocytes and neutrophils, and upregulated by toll-like receptor (TLR) ligands; activation of TREM1 amplifies immune responses.[6][8][9]
Function
[edit]Monocyte-, macrophage- and neutrophil-mediated inflammatory responses can be stimulated via receptors such as G protein-linked 7-transmembrane receptors (such as FPR1), Fc receptors, CD14, TLRs (such as TLR4), and cytokine receptors. TREM1 amplifies TLR-induced inflammation by increasing production of inflammatory cytokines.[10] The ligand of TREM1 is unknown, however, bacterial infection, ischemic stroke and challenge with lipopolysaccharide or lipoteichoic acid were observed to increase TREM1 expression.[8] In granulocytes, C/EBPε induces TREM1 expression independently from inflammatory response.[11]
TREM1 forms a complex with transmembrane adaptor DAP12 and, upon TREM1 engagement, a protein tyrosine kinase Syk-mediated cascade of tyrosine phosphorylation is initiated, activating downstream mediators such as PLCγ, PI3K, and MAPK. This cascade promotes the release of inflammatory cytokines and/or chemokines by neutrophils and macrophages, as well as their migration.[12][13]
Based on laboratory studies, TREM1 might be involved in development of atherosclerosis,[14] non-alcoholic fatty liver disease (NAFLD),[15] and ischemic stroke.[16]
Cancer
[edit]TREM1 expression is higher in tumor vs non-tumor tissues, likely due to its expression by myeloid cells that infiltrate tumors.[17][18] TREM1 is expressed by myeloid immune-suppressive cell populations such as monocytic myeloid-derived suppressor cells (mMDSC), tumor-associated neutrophils (TANs), and tumor associated macrophages (TAMs). These types of tumor-associated myeloid infiltrate correlate with shorter survival times of patients with solid tumors, and are likely to be the reason that not all tumors respond to checkpoint inhibitor therapy.[19][20] Antibodies that target TREM1 are therefore being studied for use in treatment patients with tumors that do not respond to checkpoint inhibitor therapy.
Flow cytometric analyses of human tumor-infiltrating myeloid cells isolated from breast cancer, bladder cancer, endometrial cancer, head and neck squamous cell carcinoma, ovarian cancer, prostate cancer, and renal cell carcinoma tissues demonstrated that within tumors, TREM1 is enriched on myeloid subsets, including mMDSCs, TANs, TAMs (AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics 2021 abstract no. P104). Single-cell immune profiling of stage III-C ovarian tumor specimens revealed that TREM1 is specifically expressed in TAM and monocytes in these tumors (AACR-NCI-EORTC INTERNATIONAL CONFERENCE ON MOLECULAR TARGETS AND CANCER THERAPEUTICS 2019 abstract no. C105). Additional studies found high expression of TREM1 protein and TREM1 mRNA, respectively, in TAMs and monocytes in human tumors (Immunotherapy of Cancer Conference (ITOC) 2021 Abstract P02.11, Society for the Immunotherapy of Cancer (SITC) Conference 2021, Poster 859).
Higher levels of TREM1 mRNA in tumor tissues correlate with shorter survival times of patients with colon cancer, breast cancer, pancreatic cancer, and squamous cell carcinoma (AACR-NCI-EORTC INTERNATIONAL CONFERENCE ON MOLECULAR TARGETS AND CANCER THERAPEUTICS 2019 abstract no. C105).
Soluble TREM1
[edit]During inflammation, a soluble form of the molecule (sTREM1) accumulates in the circulation, and is a biomarker of inflammation. There is debate over whether sTREM1 is produced as a splice variant or as the results of proteolytic cleavage.[21][22] sTREM1 acts as a decoy for the unknown TREM1 ligands, and thereby prevents TREM1 activation.[23] sTREM1 has been studied as a biomarker of development of pneumonia, sepsis,[24] inflammatory bowel diseases,[25][26] and liver cirrhosis.[27]
Model organisms
[edit]Model organisms have been used in the study of TREM1 function. A conditional knockout mouse line called Trem1tm1(KOMP)Vlcg was generated at the Wellcome Trust Sanger Institute.[28] Male and female animals underwent a standardized phenotypic screen[29] to determine the effects of deletion.[30][31][32][33] Additional screens included immune phenotyping.[34] Blockade of TREM1 protects mice against microbe-induced shock, indicating that it is an important regulator of the immune response.[8]
TREM1 as a Therapeutic Target
[edit]Pionyr Immunotherapeutics is developing PY159 for treatment of advanced solid tumors refractory to or relapsed with the current standard of care. PY159 is a humanized monoclonal antibody that binds TREM1 and acts as an agonist, crosslinking TREM1 to induce signaling through the TREM1-DAP12 complex (SITC 2019 Poster P812, AACR-NCI-EORTC INTERNATIONAL CONFERENCE ON MOLECULAR TARGETS AND CANCER THERAPEUTICS 2019 abstract no. C-105, AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics 2021 Abstract P104, ITOC 2021 Abstract P02.11, SITC 2021 Poster 859). PY159 is being evaluated in a phase 1 trial, in combination with pembrolizumab, in subjects with advanced solid tumors
Celsius Therapeutics is developing CEL383, an antibody inhibitor of TREM1, for treatment of inflammatory bowel disease. As of October 2023, CEL383 is being evaluated in a phase 1 trial in healthy volunteers.
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000124731 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000042265 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Gingras MC, Lapillonne H, Margolin JF (March 2002). "TREM-1, MDL-1, and DAP12 expression is associated with a mature stage of myeloid development". Molecular Immunology. 38 (11): 817–24. doi:10.1016/S0161-5890(02)00004-4. PMID 11922939.
- ^ a b Bouchon A, Dietrich J, Colonna M (May 2000). "Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes". Journal of Immunology. 164 (10): 4991–5. doi:10.4049/jimmunol.164.10.4991. PMID 10799849.
- ^ "Entrez Gene: TREM1 triggering receptor expressed on myeloid cells 1".
- ^ a b c Bouchon A, Facchetti F, Weigand MA, Colonna M (April 2001). "TREM-1 amplifies inflammation and is a crucial mediator of septic shock". Nature. 410 (6832): 1103–7. doi:10.1038/35074114. PMID 11323674. S2CID 4403589.
- ^ Mabbott NA, Baillie J, Brown H, Freeman TC, Hume DA (2013). "An expression atlas of human primary cells: inference of gene function from coexpression networks". BMC Genomics. 14 (1): 632. doi:10.1186/1471-2164-14-632. ISSN 1471-2164. PMC 3849585. PMID 24053356.
- ^ Arts RJ, Joosten LA, van der Meer JW, Netea MG (February 2013). "TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors". Journal of Leukocyte Biology. 93 (2): 209–15. doi:10.1189/jlb.0312145. PMID 23108097. S2CID 29507561.
- ^ Suh HC, Benoukraf T, Shyamsunder P, Yin T, Cao Q, Said J, et al. (April 2017). "LPS independent activation of the pro-inflammatory receptor Trem1 by C/EBPε in granulocytes". Scientific Reports. 7: 46440. Bibcode:2017NatSR...746440S. doi:10.1038/srep46440. PMC 5404328. PMID 28440307.
- ^ Niu Z, Shi Q, Zhang W, Shu Y, Yang N, Chen B, Wang Q, Zhao X, Chen J, Cheng N, Feng X (December 2017). "Caspase-1 cleaves PPARγ for potentiating the pro-tumor action of TAMs". Nature Communications. 8 (1): 766. Bibcode:2017NatCo...8..766N. doi:10.1038/s41467-017-00523-6. ISSN 2041-1723. PMC 5626701. PMID 28974683.
- ^ Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC (2017-09-01). "TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives". Pharmacology & Therapeutics. 177: 81–95. doi:10.1016/j.pharmthera.2017.02.043. hdl:2066/177974. ISSN 0163-7258. PMID 28245991. S2CID 19380728.
- ^ Zysset D, Weber B, Rihs S, Brasseit J, Freigang S, Riether C, et al. (October 2016). "TREM-1 links dyslipidemia to inflammation and lipid deposition in atherosclerosis". Nature Communications. 7 (1): 13151. Bibcode:2016NatCo...713151Z. doi:10.1038/ncomms13151. PMC 5080444. PMID 27762264.
- ^ Rao S, Huang J, Shen Z, Xiang C, Zhang M, Lu X (February 2019). "Inhibition of TREM-1 attenuates inflammation and lipid accumulation in diet-induced nonalcoholic fatty liver disease". Journal of Cellular Biochemistry. 120 (7): 11867–11877. doi:10.1002/jcb.28468. PMC 6593463. PMID 30805986.
- ^ Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC (September 2017). "TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives". Pharmacology & Therapeutics. 177: 81–95. doi:10.1016/j.pharmthera.2017.02.043. hdl:2066/177974. PMID 28245991. S2CID 19380728.
- ^ Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, Amigorena S (November 2014). "Dissecting the Tumor Myeloid Compartment Reveals Rare Activating Antigen-Presenting Cells Critical for T Cell Immunity". Cancer Cell. 26 (5): 638–652. doi:10.1016/j.ccell.2014.09.007. PMC 4254577. PMID 25446897.
- ^ Lavin Y, Kobayashi S, Leader A, Amir Ea, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, Meinhof K (May 2017). "Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses". Cell. 169 (4): 750–765.e17. doi:10.1016/j.cell.2017.04.014. PMC 5737939. PMID 28475900.
- ^ Awad RM, De Vlaeminck Y, Maebe J, Goyvaerts C, Breckpot K (2018-08-31). "Turn Back the TIMe: Targeting Tumor Infiltrating Myeloid Cells to Revert Cancer Progression". Frontiers in Immunology. 9: 1977. doi:10.3389/fimmu.2018.01977. ISSN 1664-3224. PMC 6127274. PMID 30233579.
- ^ Bingle L, Brown NJ, Lewis CE (March 2002). "The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies". The Journal of Pathology. 196 (3): 254–265. doi:10.1002/path.1027. ISSN 0022-3417. PMID 11857487. S2CID 8481685.
- ^ Baruah S, Keck K, Vrenios M, Pope MR, Pearl M, Doerschug K, Klesney-Tait J (December 2015). "Identification of a Novel Splice Variant Isoform of TREM-1 in Human Neutrophil Granules". Journal of Immunology. 195 (12): 5725–31. doi:10.4049/jimmunol.1402713. PMC 4670805. PMID 26561551.
- ^ Gómez-Piña V, Soares-Schanoski A, Rodríguez-Rojas A, Del Fresno C, García F, Vallejo-Cremades MT, et al. (September 2007). "Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes". Journal of Immunology. 179 (6): 4065–73. doi:10.4049/jimmunol.179.6.4065. PMID 17785845.
- ^ Gibot S, Kolopp-Sarda MN, Béné MC, Bollaert PE, Lozniewski A, Mory F, et al. (December 2004). "A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis". The Journal of Experimental Medicine. 200 (11): 1419–26. doi:10.1084/jem.20040708. PMC 2211948. PMID 15557347.
- ^ Tejera A, Santolaria F, Diez ML, Alemán-Valls MR, González-Reimers E, Martínez-Riera A, Milena-Abril A (June 2007). "Prognosis of community acquired pneumonia (CAP): value of triggering receptor expressed on myeloid cells-1 (TREM-1) and other mediators of the inflammatory response". Cytokine. 38 (3): 117–23. doi:10.1016/j.cyto.2007.05.002. PMID 17659879.
- ^ Park JJ, Cheon JH, Kim BY, Kim DH, Kim ES, Kim TI, et al. (July 2009). "Correlation of serum-soluble triggering receptor expressed on myeloid cells-1 with clinical disease activity in inflammatory bowel disease". Digestive Diseases and Sciences. 54 (7): 1525–31. doi:10.1007/s10620-008-0514-5. PMID 18975078. S2CID 12356021.
- ^ Jung YS, Park JJ, Kim SW, Hong SP, Kim TI, Kim WH, Cheon JH (November 2012). "Correlation between soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) expression and endoscopic activity in inflammatory bowel diseases". Digestive and Liver Disease. 44 (11): 897–903. doi:10.1016/j.dld.2012.05.011. PMID 22721842.
- ^ Tornai D, Vitalis Z, Jonas A, Janka T, Foldi I, Tornai T, et al. (March 2021). "Increased sTREM-1 levels identify cirrhotic patients with bacterial infection and predict their 90-day mortality". Clinics and Research in Hepatology and Gastroenterology. 45 (5): 101579. doi:10.1016/j.clinre.2020.11.009. PMID 33773436.
- ^ Gerdin AK (2010). "The Sanger Mouse Genetics Programme: high throughput characterisation of knockout mice". Acta Ophthalmologica. 88: 925–7. doi:10.1111/j.1755-3768.2010.4142.x. S2CID 85911512.
- ^ "International Mouse Phenotyping Consortium".
- ^ Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, et al. (June 2011). "A conditional knockout resource for the genome-wide study of mouse gene function". Nature. 474 (7351): 337–42. doi:10.1038/nature10163. PMC 3572410. PMID 21677750.
- ^ Dolgin E (June 2011). "Mouse library set to be knockout". Nature. 474 (7351): 262–3. doi:10.1038/474262a. PMID 21677718. S2CID 39281705.
- ^ Collins FS, Rossant J, Wurst W (January 2007). "A mouse for all reasons". Cell. 128 (1): 9–13. doi:10.1016/j.cell.2006.12.018. PMID 17218247. S2CID 18872015.
- ^ White JK, Gerdin AK, Karp NA, Ryder E, Buljan M, Bussell JN, et al. (July 2013). "Genome-wide generation and systematic phenotyping of knockout mice reveals new roles for many genes". Cell. 154 (2): 452–64. doi:10.1016/j.cell.2013.06.022. PMC 3717207. PMID 23870131.
- ^ "Infection and Immunity Immunophenotyping (3i) Consortium".
Further reading
[edit]- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Bouchon A, Facchetti F, Weigand MA, Colonna M (April 2001). "TREM-1 amplifies inflammation and is a crucial mediator of septic shock". Nature. 410 (6832): 1103–7. Bibcode:2001Natur.410.1103B. doi:10.1038/35074114. PMID 11323674. S2CID 4403589.
- Allcock RJ, Barrow AD, Forbes S, Beck S, Trowsdale J (February 2003). "The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44". European Journal of Immunology. 33 (2): 567–77. doi:10.1002/immu.200310033. PMID 12645956. S2CID 27147251.
- Bleharski JR, Kiessler V, Buonsanti C, Sieling PA, Stenger S, Colonna M, Modlin RL (April 2003). "A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response". Journal of Immunology. 170 (7): 3812–8. doi:10.4049/jimmunol.170.7.3812. PMID 12646648.
- Colonna M, Facchetti F (June 2003). "TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses". The Journal of Infectious Diseases. 187 (Suppl 2): S397-401. doi:10.1086/374754. PMID 12792857.
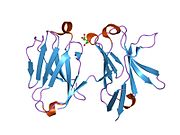
- Radaev S, Kattah M, Rostro B, Colonna M, Sun PD (December 2003). "Crystal structure of the human myeloid cell activating receptor TREM-1". Structure. 11 (12): 1527–35. doi:10.1016/j.str.2003.11.001. PMID 14656437.
- Radsak MP, Salih HR, Rammensee HG, Schild H (April 2004). "Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival". Journal of Immunology. 172 (8): 4956–63. doi:10.4049/jimmunol.172.8.4956. PMID 15067076.
- Wang DY, Qin RY, Liu ZR, Gupta MK, Chang Q (September 2004). "Expression of TREM-1 mRNA in acute pancreatitis". World Journal of Gastroenterology. 10 (18): 2744–6. doi:10.3748/wjg.v10.i18.2744. PMC 4572206. PMID 15309732.
- Kelker MS, Foss TR, Peti W, Teyton L, Kelly JW, Wüthrich K, Wilson IA (September 2004). "Crystal structure of human triggering receptor expressed on myeloid cells 1 (TREM-1) at 1.47 A". Journal of Molecular Biology. 342 (4): 1237–48. doi:10.1016/j.jmb.2004.07.089. PMID 15351648.
- Knapp S, Gibot S, de Vos A, Versteeg HH, Colonna M, van der Poll T (December 2004). "Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia". Journal of Immunology. 173 (12): 7131–4. doi:10.4049/jimmunol.173.12.7131. PMID 15585833.
- González-Roldán N, Ferat-Osorio E, Aduna-Vicente R, Wong-Baeza I, Esquivel-Callejas N, Astudillo-de la Vega H, et al. (December 2005). "Expression of triggering receptor on myeloid cell 1 and histocompatibility complex molecules in sepsis and major abdominal surgery". World Journal of Gastroenterology. 11 (47): 7473–9. doi:10.3748/wjg.v11.i47.7473. PMC 4723392. PMID 16437719.
- Tzivras M, Koussoulas V, Giamarellos-Bourboulis EJ, Tzivras D, Tsaganos T, Koutoukas P, et al. (June 2006). "Role of soluble triggering receptor expressed on myeloid cells in inflammatory bowel disease". World Journal of Gastroenterology. 12 (21): 3416–9. doi:10.3748/wjg.v12.i21.3416. PMC 4087875. PMID 16733861.
- Plachouras D, Routsi C, Giamarellos-Bourboulis EJ, Spyridaki E, Andrianakis I, Metzelopoulos S, et al. (2006). "Monocytes as a site of production of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) in the septic host". Scandinavian Journal of Infectious Diseases. 38 (10): 909–15. doi:10.1080/00365540600786523. PMID 17008237. S2CID 7754570.
- Murakami Y, Kohsaka H, Kitasato H, Akahoshi T (January 2007). "Lipopolysaccharide-induced up-regulation of triggering receptor expressed on myeloid cells-1 expression on macrophages is regulated by endogenous prostaglandin E2". Journal of Immunology. 178 (2): 1144–50. doi:10.4049/jimmunol.178.2.1144. PMID 17202378.