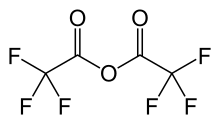
Trifluoroacetic anhydride
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Trifluoroacetic anhydride | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.006.349 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4F6O3 | |
| Molar mass | 210.031 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.511 g/mL (20°C) |
| Melting point | −65 °C (−85 °F; 208 K) |
| Boiling point | 40 °C (104 °F; 313 K) |
| reacts | |
| Solubility | soluble in benzene, dichloromethane, ether, DMF, THF, acetonitrile |
| Hazards | |
| GHS labelling: | |
  [1] [1]
| |
| Danger | |
| H314, H332[1] | |
| P280, P305+P351+P338, P310 [1] | |
| Safety data sheet (SDS) | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trifluoroacetic anhydride (TFAA) is the acid anhydride of trifluoroacetic acid. It is the perfluorinated derivative of acetic anhydride.
Preparation
[edit]Trifluoroacetic anhydride was originally prepared by the dehydration of trifluoroacetic acid with phosphorus pentoxide.[2] The dehydration might also be carried out with excess α-halogenated acid chlorides. For example, with dichloroacetyl chloride:[3]
- 2 CF3COOH + Cl2CHCOCl → (CF3CO)2O + Cl2CHCOOH + HCl
Uses
[edit]Trifluoroacetic anhydride has various uses in organic synthesis.
It may be used to introduce the corresponding trifluoroacetyl group, for which it is more convenient than the corresponding acyl chloride, trifluoroacetyl chloride, which is a gas.
It can be used to promote reactions of carboxylic acids, including Friedel-Crafts acylation and acylation of other unsaturated compounds. Other electrophilic aromatic substitution reactions can also be promoted with trifluoroacetic anhydride, including nitration, sulfonation and nitrosylation.[2]
Similar to acetic anhydride, trifluoroacetic anhydride can be used as a dehydrating agent and as an activator for the Pummerer rearrangement.[4]
It can be used in place of oxalyl chloride in the Swern oxidation, allowing temperatures up to −30 °C.[5]
With sodium iodide, it reduces sulfoxides to sulfides.[4]
Trifluoroacetic anhydride is the recommended desiccant for trifluoroacetic acid.[6]
References
[edit]- ^ a b c Sigma-Aldrich Co., Trifluoroacetic Anhydride. Retrieved on 2020-06-08.
- ^ a b Tedder, J. M. (1955). "The Use of Trifluoroacetic Anhydride and Related Compounds in Organic Synthesis". Chem. Rev. 55 (5): 787–827. doi:10.1021/cr50005a001.
- ^ US 4595541, Amiet, Louis & Disdier, Camille, "Process for the preparation of trifluoroacetic anhydride", published 1986-06-17, assigned to Rhone Poulenc Specialites Chimiques
- ^ a b Sweeney, Joseph; Perkins, Gemma; DiMauro, Erin F.; Hodous, Brian L. (2005). "Trifluoroacetic Anhydride". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rt237.pub2. ISBN 978-0-470-84289-8.
- ^ Omura, Kanji; Sharma, Ashok K.; Swern, Daniel (1976). "Dimethyl Sulfoxide-Trifluoroacetic Anhydride. New Reagent for Oxidation of Alcohols to Carbonyls". J. Org. Chem. 41 (6): 957–962. doi:10.1021/jo00868a012.
- ^ Chai, Christina Li Lin; Armarego, W. L. F. (2003). Purification of laboratory chemicals (Google Books excerpt). Oxford: Butterworth-Heinemann. p. 376. ISBN 0-7506-7571-3.
