Chemical synthesis
This article needs additional citations for verification. (March 2016) |
Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products.[1] This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable.
A chemical synthesis involves one or more compounds (known as reagents or reactants) that will experience a transformation under certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing ("work-up") or purification procedure to isolate the final product.[1]
The amount produced by chemical synthesis is known as the reaction yield. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that could be produced based on the limiting reagent. A side reaction is an unwanted chemical reaction that can reduce the desired yield. The word synthesis was used first in a chemical context by the chemist Hermann Kolbe.[2]
Strategies
[edit]Many strategies exist in chemical synthesis that are more complicated than simply converting a reactant A to a reaction product B directly. For multistep synthesis, a chemical compound is synthesized by a series of individual chemical reactions, each with its own work-up.[3] For example, a laboratory synthesis of paracetamol can consist of three sequential parts. For cascade reactions, multiple chemical transformations occur within a single reactant, for multi-component reactions as many as 11 different reactants form a single reaction product and for a "telescopic synthesis" one reactant experiences multiple transformations without isolation of intermediates.
Organic synthesis
[edit]Organic synthesis is a special type of chemical synthesis dealing with the synthesis of organic compounds. For the total synthesis of a complex product, multiple procedures in sequence may be required to synthesize the product of interest, needing a lot of time. A purely synthetic chemical synthesis begins with basic lab compounds. A semisynthetic process starts with natural products from plants or animals and then modifies them into new compounds.
Inorganic synthesis
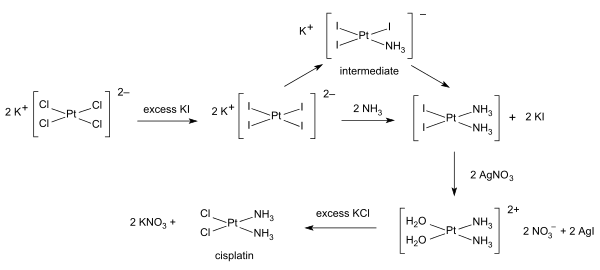
[edit]Inorganic synthesis and organometallic synthesis are used to prepare compounds with significant non-organic content. An illustrative example is the preparation of the anti-cancer drug cisplatin from potassium tetrachloroplatinate.[4]
See also
[edit]References
[edit]- ^ a b Vogel, A.I.; Tatchell, A.R.; Furnis, B.S.; Hannaford, A.J.; Smith, P.W.G. (1996). Vogel's Textbook of Practical Organic Chemistry (5th ed.). Prentice Hall. ISBN 0-582-46236-3.
- ^ Kolbe, H. (1845). "Beiträge zur Kenntniss der gepaarten Verbindungen". Annalen der Chemie und Pharmacie. 54 (2): 145–188. doi:10.1002/jlac.18450540202. ISSN 0075-4617. Archived from the original on Jun 30, 2023 – via Zenodo.
- ^ Carey, Francis A.; Sundberg, Richard J. (2013). Advanced Organic Chemistry Part B: Reactions and Synthesis. Springer.
- ^ Alderden, Rebecca A.; Hall, Matthew D.; Hambley, Trevor W. (1 May 2006). "The Discovery and Development of Cisplatin". J. Chem. Educ. 83 (5): 728. Bibcode:2006JChEd..83..728A. doi:10.1021/ed083p728.
