Genetic disorder
| Genetic disorder | |
|---|---|
 | |
| Diagram featuring examples of a disease located on each chromosome | |
| Specialty | Medical genetics |
A genetic disorder is a health problem caused by one or more abnormalities in the genome. It can be caused by a mutation in a single gene (monogenic) or multiple genes (polygenic) or by a chromosome abnormality. Although polygenic disorders are the most common, the term is mostly used when discussing disorders with a single genetic cause, either in a gene or chromosome.[1][2] The mutation responsible can occur spontaneously before embryonic development (a de novo mutation), or it can be inherited from two parents who are carriers of a faulty gene (autosomal recessive inheritance) or from a parent with the disorder (autosomal dominant inheritance). When the genetic disorder is inherited from one or both parents, it is also classified as a hereditary disease. Some disorders are caused by a mutation on the X chromosome and have X-linked inheritance. Very few disorders are inherited on the Y chromosome or mitochondrial DNA (due to their size).[3]
There are well over 6,000 known genetic disorders,[4] and new genetic disorders are constantly being described in medical literature.[5] More than 600 genetic disorders are treatable.[6] Around 1 in 50 people are affected by a known single-gene disorder, while around 1 in 263 are affected by a chromosomal disorder.[7] Around 65% of people have some kind of health problem as a result of congenital genetic mutations.[7] Due to the significantly large number of genetic disorders, approximately 1 in 21 people are affected by a genetic disorder classified as "rare" (usually defined as affecting less than 1 in 2,000 people). Most genetic disorders are rare in themselves.[5][8]
Genetic disorders are present before birth, and some genetic disorders produce birth defects, but birth defects can also be developmental rather than hereditary. The opposite of a hereditary disease is an acquired disease. Most cancers, although they involve genetic mutations to a small proportion of cells in the body, are acquired diseases. Some cancer syndromes, however, such as BRCA mutations, are hereditary genetic disorders.[9]
Single-gene
[edit]| Disorder prevalence (approximate) | |
|---|---|
| Autosomal dominant | |
| Familial hypercholesterolemia | 1 in 500[11] |
| Myotonic dystrophy type 1 | 1 in 2,100[12] |
| Neurofibromatosis type I | 1 in 2,500[13] |
| Hereditary spherocytosis | 1 in 5,000 |
| Marfan syndrome | 1 in 4,000[14] |
| Huntington's disease | 1 in 15,000[15] |
| Autosomal recessive | |
| Sickle cell anaemia | 1 in 625[16] |
| Cystic fibrosis | 1 in 2,000 |
| Tay–Sachs disease | 1 in 3,000 |
| Phenylketonuria | 1 in 12,000 |
| Autosomal recessive polycystic kidney disease | 1 in 20,000[17] |
| Mucopolysaccharidoses | 1 in 25,000 |
| Lysosomal acid lipase deficiency | 1 in 40,000 |
| Glycogen storage diseases | 1 in 50,000 |
| Galactosemia | 1 in 57,000 |
| X-linked | |
| Duchenne muscular dystrophy | 1 in 5,000 |
| Hemophilia | 1 in 10,000 |
| Values are for liveborn infants | |
A single-gene disorder (or monogenic disorder) is the result of a single mutated gene. Single-gene disorders can be passed on to subsequent generations in several ways. Genomic imprinting and uniparental disomy, however, may affect inheritance patterns. The divisions between recessive and dominant types are not "hard and fast", although the divisions between autosomal and X-linked types are (since the latter types are distinguished purely based on the chromosomal location of the gene). For example, the common form of dwarfism, achondroplasia, is typically considered a dominant disorder, but children with two genes for achondroplasia have a severe and usually lethal skeletal disorder, one that achondroplasics could be considered carriers for. Sickle cell anemia is also considered a recessive condition, but heterozygous carriers have increased resistance to malaria in early childhood, which could be described as a related dominant condition.[18] When a couple where one partner or both are affected or carriers of a single-gene disorder wish to have a child, they can do so through in vitro fertilization, which enables preimplantation genetic diagnosis to occur to check whether the embryo has the genetic disorder.[19]
Most congenital metabolic disorders known as inborn errors of metabolism result from single-gene defects. Many such single-gene defects can decrease the fitness of affected people and are therefore present in the population in lower frequencies compared to what would be expected based on simple probabilistic calculations.[20]
Autosomal dominant
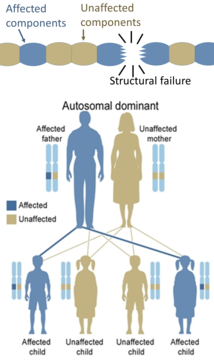
[edit]Only one mutated copy of the gene will be necessary for a person to be affected by an autosomal dominant disorder. Each affected person usually has one affected parent.[21]: 57 The chance a child will inherit the mutated gene is 50%. Autosomal dominant conditions sometimes have reduced penetrance, which means although only one mutated copy is needed, not all individuals who inherit that mutation go on to develop the disease. Examples of this type of disorder are Huntington's disease,[21]: 58 neurofibromatosis type 1, neurofibromatosis type 2, Marfan syndrome, hereditary nonpolyposis colorectal cancer, hereditary multiple exostoses (a highly penetrant autosomal dominant disorder), tuberous sclerosis, Von Willebrand disease, and acute intermittent porphyria. Birth defects are also called congenital anomalies.[22]
Autosomal recessive
[edit]Two copies of the gene must be mutated for a person to be affected by an autosomal recessive disorder. An affected person usually has unaffected parents who each carry a single copy of the mutated gene and are referred to as genetic carriers. Each parent with a defective gene normally do not have symptoms.[23] Two unaffected people who each carry one copy of the mutated gene have a 25% risk with each pregnancy of having a child affected by the disorder. Examples of this type of disorder are albinism, medium-chain acyl-CoA dehydrogenase deficiency, cystic fibrosis, sickle cell disease, Tay–Sachs disease, Niemann–Pick disease, spinal muscular atrophy, and Roberts syndrome. Certain other phenotypes, such as wet versus dry earwax, are also determined in an autosomal recessive fashion.[24][25] Some autosomal recessive disorders are common because, in the past, carrying one of the faulty genes led to a slight protection against an infectious disease or toxin such as tuberculosis or malaria.[26] Such disorders include cystic fibrosis,[27] sickle cell disease,[28] phenylketonuria[29] and thalassaemia.[30]
-
Hereditary defects in enzymes are generally inherited in an autosomal fashion because there are more non-X chromosomes than X-chromosomes, and a recessive fashion because the enzymes from the unaffected genes are generally sufficient to prevent symptoms in carriers.
-
On the other hand, hereditary defects in structural proteins (such as osteogenesis imperfecta, Marfan's syndrome and many Ehlers–Danlos syndromes) are generally autosomal dominant, because it is enough that some components are defective to make the whole structure dysfunctional. This is a dominant-negative process, wherein a mutated gene product adversely affects the non-mutated gene product within the same cell.
X-linked dominant
[edit]
X-linked dominant disorders are caused by mutations in genes on the X chromosome. Only a few disorders have this inheritance pattern, with a prime example being X-linked hypophosphatemic rickets. Males and females are both affected in these disorders, with males typically being more severely affected than females. Some X-linked dominant conditions, such as Rett syndrome, incontinentia pigmenti type 2, and Aicardi syndrome, are usually fatal in males either in utero or shortly after birth, and are therefore predominantly seen in females. Exceptions to this finding are extremely rare cases in which boys with Klinefelter syndrome (44+xxy) also inherit an X-linked dominant condition and exhibit symptoms more similar to those of a female in terms of disease severity. The chance of passing on an X-linked dominant disorder differs between men and women. The sons of a man with an X-linked dominant disorder will all be unaffected (since they receive their father's Y chromosome), but his daughters will all inherit the condition. A woman with an X-linked dominant disorder has a 50% chance of having an affected foetus with each pregnancy, although in cases such as incontinentia pigmenti, only female offspring are generally viable.
X-linked recessive
[edit]X-linked recessive conditions are also caused by mutations in genes on the X chromosome. Males are much more frequently affected than females, because they only have the one X chromosome necessary for the condition to present. The chance of passing on the disorder differs between men and women. The sons of a man with an X-linked recessive disorder will not be affected (since they receive their father's Y chromosome), but his daughters will be carriers of one copy of the mutated gene. A woman who is a carrier of an X-linked recessive disorder (XRXr) has a 50% chance of having sons who are affected and a 50% chance of having daughters who are carriers of one copy of the mutated gene. X-linked recessive conditions include the serious diseases hemophilia A, Duchenne muscular dystrophy, and Lesch–Nyhan syndrome, as well as common and less serious conditions such as male pattern baldness and red–green color blindness. X-linked recessive conditions can sometimes manifest in females due to skewed X-inactivation or monosomy X (Turner syndrome).[citation needed]
Y-linked
[edit]Y-linked disorders are caused by mutations on the Y chromosome. These conditions may only be transmitted from the heterogametic sex (e.g. male humans) to offspring of the same sex. More simply, this means that Y-linked disorders in humans can only be passed from men to their sons; females can never be affected because they do not possess Y-allosomes.[citation needed]
Y-linked disorders are exceedingly rare but the most well-known examples typically cause infertility. Reproduction in such conditions is only possible through the circumvention of infertility by medical intervention.
Mitochondrial
[edit]This type of inheritance, also known as maternal inheritance, is the rarest and applies to the 13 genes encoded by mitochondrial DNA. Because only egg cells contribute mitochondria to the developing embryo, only mothers (who are affected) can pass on mitochondrial DNA conditions to their children. An example of this type of disorder is Leber's hereditary optic neuropathy.[31]
It is important to stress that the vast majority of mitochondrial diseases (particularly when symptoms develop in early life) are actually caused by a nuclear gene defect, as the mitochondria are mostly developed by non-mitochondrial DNA. These diseases most often follow autosomal recessive inheritance.[32]
Multifactorial disorder
[edit]Genetic disorders may also be complex, multifactorial, or polygenic, meaning they are likely associated with the effects of multiple genes in combination with lifestyles and environmental factors. Multifactorial disorders include heart disease and diabetes. Although complex disorders often cluster in families, they do not have a clear-cut pattern of inheritance. This makes it difficult to determine a person's risk of inheriting or passing on these disorders. Complex disorders are also difficult to study and treat because the specific factors that cause most of these disorders have not yet been identified. Studies that aim to identify the cause of complex disorders can use several methodological approaches to determine genotype–phenotype associations. One method, the genotype-first approach, starts by identifying genetic variants within patients and then determining the associated clinical manifestations. This is opposed to the more traditional phenotype-first approach, and may identify causal factors that have previously been obscured by clinical heterogeneity, penetrance, and expressivity.[citation needed]
On a pedigree, polygenic diseases do tend to "run in families", but the inheritance does not fit simple patterns as with Mendelian diseases. This does not mean that the genes cannot eventually be located and studied. There is also a strong environmental component to many of them (e.g., blood pressure).
Other such cases include:
- asthma
- autoimmune diseases such as multiple sclerosis
- cancers
- ciliopathies
- cleft palate
- diabetes
- heart disease
- hypertension
- inflammatory bowel disease
- intellectual disability
- mood disorder
- obesity
- refractive error
- infertility
Chromosomal disorder
[edit]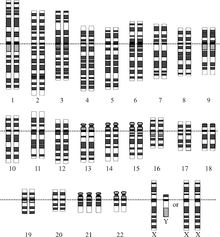
A chromosomal disorder is a missing, extra, or irregular portion of chromosomal DNA.[33] It can be from an atypical number of chromosomes or a structural abnormality in one or more chromosomes. An example of these disorders is Trisomy 21 (the most common form of Down syndrome), in which there is an extra copy of chromosome 21 in all cells.[34]
Diagnosis
[edit]Due to the wide range of genetic disorders that are known, diagnosis is widely varied and dependent of the disorder. Most genetic disorders are diagnosed pre-birth, at birth, or during early childhood however some, such as Huntington's disease, can escape detection until the patient begins exhibiting symptoms well into adulthood.[35]
The basic aspects of a genetic disorder rests on the inheritance of genetic material. With an in depth family history, it is possible to anticipate possible disorders in children which direct medical professionals to specific tests depending on the disorder and allow parents the chance to prepare for potential lifestyle changes, anticipate the possibility of stillbirth, or contemplate termination.[36] Prenatal diagnosis can detect the presence of characteristic abnormalities in fetal development through ultrasound, or detect the presence of characteristic substances via invasive procedures which involve inserting probes or needles into the uterus such as in amniocentesis.[37]
Prognosis
[edit]Not all genetic disorders directly result in death; however, there are no known cures for genetic disorders. Many genetic disorders affect stages of development, such as Down syndrome, while others result in purely physical symptoms such as muscular dystrophy. Other disorders, such as Huntington's disease, show no signs until adulthood. During the active time of a genetic disorder, patients mostly rely on maintaining or slowing the degradation of quality of life and maintain patient autonomy. This includes physical therapy and pain management.
Treatment
[edit]
The treatment of genetic disorders is an ongoing battle, with over 1,800 gene therapy clinical trials having been completed, are ongoing, or have been approved worldwide.[38] Despite this, most treatment options revolve around treating the symptoms of the disorders in an attempt to improve patient quality of life.
Gene therapy refers to a form of treatment where a healthy gene is introduced to a patient. This should alleviate the defect caused by a faulty gene or slow the progression of the disease. A major obstacle has been the delivery of genes to the appropriate cell, tissue, and organ affected by the disorder. Researchers have investigated how they can introduce a gene into the potentially trillions of cells that carry the defective copy. Finding an answer to this has been a roadblock between understanding the genetic disorder and correcting the genetic disorder.[39]
Epidemiology
[edit]Around 1 in 50 people are affected by a known single-gene disorder, while around 1 in 263 are affected by a chromosomal disorder.[7] Around 65% of people have some kind of health problem as a result of congenital genetic mutations.[7] Due to the significantly large number of genetic disorders, approximately 1 in 21 people are affected by a genetic disorder classified as "rare" (usually defined as affecting less than 1 in 2,000 people). Most genetic disorders are rare in themselves.[5][8] There are well over 6,000 known genetic disorders,[4] and new genetic disorders are constantly being described in medical literature.[5]
History
[edit]The earliest known genetic condition in a hominid was in the fossil species Paranthropus robustus, with over a third of individuals displaying amelogenesis imperfecta.[40]
See also
[edit]- FINDbase (the Frequency of Inherited Disorders database)
- Genetic epidemiology
- List of genetic disorders
- Population groups in biomedicine
- Mendelian error
References
[edit]- ^ "Genetic Disorders". Learn.Genetics. University of Utah. Archived from the original on 2022-07-15.
- ^ Lvovs D, Favorova OO, Favorov AV (July 2012). "A Polygenic Approach to the Study of Polygenic Diseases". Acta Naturae. 4 (3): 59–71. doi:10.32607/20758251-2012-4-3-59-71. PMC 3491892. PMID 23150804.
- ^ "What are the different ways in which a genetic condition can be inherited?". Genetics Home Reference. Archived from the original on 2020-09-27. Retrieved 2020-01-14.
- ^ a b "OMIM Gene Map Statistics". OMIM. Archived from the original on 2020-01-28. Retrieved 2020-01-14.
- ^ a b c d "About rare diseases". Orphanet. Archived from the original on 2019-12-17. Retrieved 2020-01-14.
- ^ Bick D, Bick SL, Dimmock DP, Fowler TA, Caulfield MJ, Scott RH (March 2021). "An online compendium of treatable genetic disorders". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 187 (1): 48–54. doi:10.1002/ajmg.c.31874. PMC 7986124. PMID 33350578.
- ^ a b c d Kumar P, Radhakrishnan J, Chowdhary MA, Giampietro PF (August 2001). "Prevalence and patterns of presentation of genetic disorders in a pediatric emergency department". Mayo Clinic Proceedings. 76 (8): 777–783. doi:10.4065/76.8.777. PMID 11499815.
- ^ a b Jackson M, Marks L, May GH, Wilson JB (December 2018). "The genetic basis of disease". Essays in Biochemistry. 62 (5): 643–723. doi:10.1042/EBC20170053. PMC 6279436. PMID 30509934.
(calculated from "1 in 17" rare disorders and "80%" of rare disorders being genetic)
- ^ Hunt JD. "An Introduction to Cancer". Genetics and Louisiana Families. lsuhsc.edu. Archived from the original on 16 January 2020.
- ^ "Prevalence and incidence of rare diseases" (PDF). Archived (PDF) from the original on 2008-11-18.
- ^ "OMIM Entry #144010 – HYPERCHOLESTEROLEMIA, FAMILIAL, 2; FCHL2". omim.org. Archived from the original on 2021-03-09. Retrieved 2019-07-01.
- ^ Johnson NE, Butterfield RJ, Mayne K, Newcomb T, Imburgia C, Dunn D, et al. (February 2021). "Population-Based Prevalence of Myotonic Dystrophy Type 1 Using Genetic Analysis of Statewide Blood Screening Program". Neurology. 96 (7): e1045–e1053. doi:10.1212/WNL.0000000000011425. PMC 8055332. PMID 33472919.
- ^ "OMIM Entry #162200 – NEUROFIBROMATOSIS, TYPE I; NF1". omim.org. Archived from the original on 2021-03-08. Retrieved 2019-07-01.
- ^ Keane MG, Pyeritz RE (May 2008). "Medical management of Marfan syndrome". Circulation. 117 (21): 2802–2813. doi:10.1161/CIRCULATIONAHA.107.693523. PMID 18506019.
- ^ Walker FO (January 2007). "Huntington's disease". Lancet. 369 (9557): 218–228. doi:10.1016/S0140-6736(07)60111-1. PMID 17240289. S2CID 46151626.
- ^ "OMIM Entry #603903 – SICKLE CELL ANEMIA". omim.org. Archived from the original on 2021-04-26. Retrieved 2019-07-01.
- ^ Swanson K (November 2021). "Autosomal recessive polycystic kidney disease". American Journal of Obstetrics and Gynecology. 225 (5). Elsevier BV: B7–B8. doi:10.1016/j.ajog.2021.06.038. PMID 34507795. S2CID 237480065.
- ^ Williams TN, Obaro SK (July 2011). "Sickle cell disease and malaria morbidity: a tale with two tails". Trends in Parasitology. 27 (7): 315–320. doi:10.1016/j.pt.2011.02.004. PMID 21429801.
- ^ Kuliev A, Verlinsky Y (April 2005). "Preimplantation diagnosis: a realistic option for assisted reproduction and genetic practice". Current Opinion in Obstetrics & Gynecology. 17 (2): 179–183. doi:10.1097/01.gco.0000162189.76349.c5. PMID 15758612. S2CID 9382420.
- ^ Šimčíková D, Heneberg P (December 2019). "Refinement of evolutionary medicine predictions based on clinical evidence for the manifestations of Mendelian diseases". Scientific Reports. 9 (1): 18577. Bibcode:2019NatSR...918577S. doi:10.1038/s41598-019-54976-4. PMC 6901466. PMID 31819097.
- ^ a b Griffiths AJ, Wessler SR, Carroll SB, Doebley J (2012). "2: Single-Gene Inheritance". Introduction to Genetic Analysis (10th ed.). New York: W.H. Freeman and Company. ISBN 978-1-4292-2943-2.
- ^ Malherbe HL, Modell B, Blencowe H, Strong KL, Aldous C (June 2023). "A review of key terminology and definitions used for birth defects globally". Journal of Community Genetics. 14 (3): 241–262. doi:10.1007/s12687-023-00642-2. PMC 10272040. PMID 37093545.
- ^ "Inheritance Patterns for Single Gene Disorders". learn.genetics.utah.edu. Archived from the original on 2019-07-01. Retrieved 2019-07-01.
- ^ Wade N (29 January 2006). "Japanese Scientists Identify Ear Wax Gene". The New York Times. Archived from the original on 21 March 2023. Retrieved 20 February 2023.
- ^ Yoshiura K, Kinoshita A, Ishida T, Ninokata A, Ishikawa T, Kaname T, et al. (March 2006). "A SNP in the ABCC11 gene is the determinant of human earwax type". Nature Genetics. 38 (3): 324–330. doi:10.1038/ng1733. PMID 16444273. S2CID 3201966.
- ^ Mitton JB (2002). "Heterozygous Advantage". eLS. doi:10.1038/npg.els.0001760. ISBN 978-0-470-01617-6.
- ^ Poolman EM, Galvani AP (February 2007). "Evaluating candidate agents of selective pressure for cystic fibrosis". Journal of the Royal Society, Interface. 4 (12): 91–98. doi:10.1098/rsif.2006.0154. PMC 2358959. PMID 17015291.
- ^ Allison AC (October 2009). "Genetic control of resistance to human malaria". Current Opinion in Immunology. 21 (5): 499–505. doi:10.1016/j.coi.2009.04.001. PMID 19442502.
- ^ Woolf LI (May 1986). "The heterozygote advantage in phenylketonuria". American Journal of Human Genetics. 38 (5): 773–775. PMC 1684820. PMID 3717163.
- ^ Weatherall DJ (2015). "The Thalassemias: Disorders of Globin Synthesis". Williams Hematology (9e ed.). McGraw Hill Professional. p. 725. ISBN 978-0-07-183301-1. Archived from the original on 2023-02-20. Retrieved 2023-02-20.
- ^ Shemesh A, Sood G, Margolin E. "Leber Hereditary Optic Neuropathy (LHON)". StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- ^ Nussbaum R, McInnes R, Willard H (2007). Thompson & Thompson Genetics in Medicine. Philadelphia PA: Saunders. pp. 144, 145, 146. ISBN 978-1-4160-3080-5.
- ^ "Genetic Disorders: What Are They, Types, Symptoms & Causes". Cleveland Clinic. Archived from the original on 2023-11-01. Retrieved 2023-11-01.
- ^ CDC (2023-10-10). "Facts about Down Syndrome | CDC". Centers for Disease Control and Prevention. Archived from the original on 2017-07-28. Retrieved 2023-11-01.
- ^ Wyant KJ, Ridder AJ, Dayalu P (April 2017). "Huntington's Disease-Update on Treatments". Current Neurology and Neuroscience Reports. 17 (4): 33. doi:10.1007/s11910-017-0739-9. PMID 28324302.
- ^ Milunsky A, Milunsky JM (2021). "Genetic Counseling: Preconception, Prenatal, and Perinatal". Genetic Disorders and the Fetus. pp. 1–101. doi:10.1002/9781119676980.ch1. ISBN 978-1-119-67698-0.
- ^ "Diagnostic Tests – Amniocentesis". Harvard Medical School. Archived from the original on 2008-05-16. Retrieved 2008-07-15.
- ^ Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J (February 2013). "Gene therapy clinical trials worldwide to 2012 - an update". The Journal of Gene Medicine. 15 (2): 65–77. doi:10.1002/jgm.2698. PMID 23355455. S2CID 37123019.
- ^ Verma IM (August 2013). "Medicine. Gene therapy that works". Science. 341 (6148): 853–855. Bibcode:2013Sci...341..853V. doi:10.1126/science.1242551. PMID 23970689. S2CID 206550787.
- ^ Towle I, Irish JD (April 2019). "A probable genetic origin for pitting enamel hypoplasia on the molars of Paranthropus robustus" (PDF). Journal of Human Evolution. 129: 54–61. doi:10.1016/j.jhevol.2019.01.002. PMID 30904040. S2CID 85502058. Archived (PDF) from the original on 2023-06-04. Retrieved 2023-02-20.
External links
[edit]- Public Health Genomics at CDC
- OMIM — Online Mendelian Inheritance in Man, a catalog of human genes and genetic disorders
- Genetic and Rare Diseases Information Center (GARD) Office of Rare Diseases (ORD), National Institutes of Health (NIH)
- CDC's National Center on Birth Defects and Developmental Disabilities
- Genetic Disease Information from the Human Genome Project
- Global Genes Project, Genetic and Rare Diseases Organization
- List of Genetic Disorders - Genome.gov